Evaluation of Neutralizing Activity
Creative Biolabs has been working in the field of immune analysis and immunogenicity testing for many years. We have successfully provided numerous immunogenicity assessment services for worldwide customers. Now, we are happy to introduce our neutralizing activity evaluation service.
Neutralizing Antibody
Biological drugs, such as antibodies, peptides, and recombinant proteins, have the potential to induce numerous immune responses within the body. And even cause allergic or anaphylactic reactions, reduction in efficacy, or induction of autoimmunity, ranging from very minor to life-threatening. Among these adverse effects, one critical adverse immune response is the generation of neutralizing antibodies (NAbs), which could bind to the biological drug product administrated and block it perform its intended biologic function. Significantly, neutralizing antibodies could neutralize the function of the drug thereby negatively impacting the efficacy of the drug.
Evaluation of Neutralizing Activity
The assessment and characterization of NAbs have become an integral part of a drug development program for biological therapeutics. The presence of NAbs may be detected using several immunoassay methods, for example, colorimetric enzyme-linked immunosorbent assay (ELISA), immunofluorescence-based receptor binding assays, competitive ligand binding immunoassay (CLB), soluble and solid phase radioimmunoassays, and sensor-based assay. NAbs can also be detected using cell-based assays in which the presence of NAbs can be detected by their ability to inhibit the biological action of the biotherapeutic agent, for example, modulation of a biological process in the target cell.
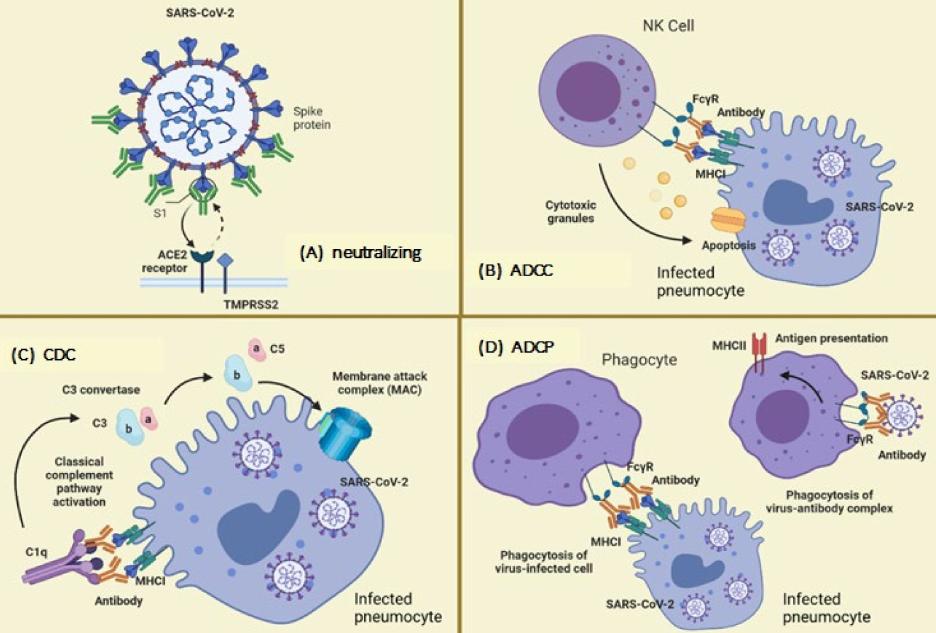 Fig.1 Neutralizing and non-neutralizing actions of antibodies.1, 2
Fig.1 Neutralizing and non-neutralizing actions of antibodies.1, 2
Neutralizing Activity Evaluation Service Provided by Creative Biolabs
During the past years, cell-based neutralizing antibody assays have been the industry's preferred method to detect NAbs as they could best mimic the mechanism of NAbs in a living biological system. With years of experience and professional scientists, Creative Biolabs has established an advanced technology platform for ADA neutralizing activity assessment. Our specialized scientists will help you to choose the most suitable stable cell line that yields adequate signal to noise responses and appropriate specificity to the drug product. In addition to cell-based assay, we also provide other immunoassay methods as an alternative approach, including but not limited to competitive ligand binding immunoassay.
Creative Biolabs is committed to providing a tiered approach for immunogenicity assessment. Aided by experienced technical team, we are able to characterize the potential neutralizing capacity, binding affinity, isotyping, and other characteristics of ADAs. In addition to neutralizing activity evaluation service, we also offer other standard and customized services to meet every client's requirements. If you are interested in the services we provide, please feel free to contact us for more information.
References
- Morales-Núñez, José Javier, et al. "Overview of neutralizing antibodies and their potential in COVID-19." Vaccines 9.12 (2021): 1376.
- Under Open Access license CC BY 4.0, without modification.
For Research Use Only.
