ADA Screening Assays
Based on the comprehensive and high-efficient platform, Creative Biolabs is professional in discovery potential immunogenicity of biological drugs. Our years of experience, vast expertise and advanced platform in immunogenicity evaluation make us confident in offering the best quality ADA screening assays for global customers.
Introduction of ADA Screening Assays
In accordance with regulatory guideline requirements, bioanalytical testing for ADAs should be conducted in a tiered testing format. Among these tiered testing methods, screening assays are the first step in any immunogenicity evaluation. The aim of the assays is to detect antibodies in patient samples which are unique to each patient and differ in characteristics (isotype and affinities etc.) between patients and also within the same patient following multiple administrations of the product. This is because different subjects may produce either one or more antibodies to a drug, each having different binding affinities and/or specificities, therefore making it impossible to compare the response to a reference standard and thus provide an accurate quantitative value.
ADA Screening Assays Provided by Creative Biolabs
With years of experience, Creative Biolabs is able to provide multiple ADA screening assay formats with high reliability and sensitivity for the quasi-quantitative or qualitative of potential antibodies. Generally, assay formats vary between different proteins and likely to depend on the therapeutic protein class. Our professional scientists will help you select an appropriate assay format to detect antibodies with desired specificities (IgM, IgG subclasses etc.) against test drugs. The common methods we offer for ADA screening are listed as follow:
- Direct/Indirect ELISA
- Bridging ELISA
- Electrochemiluminescence (ECL)
- Radioimmunoprecipitation assay (RIPA)
- Surface plasmon resonance (SPR)
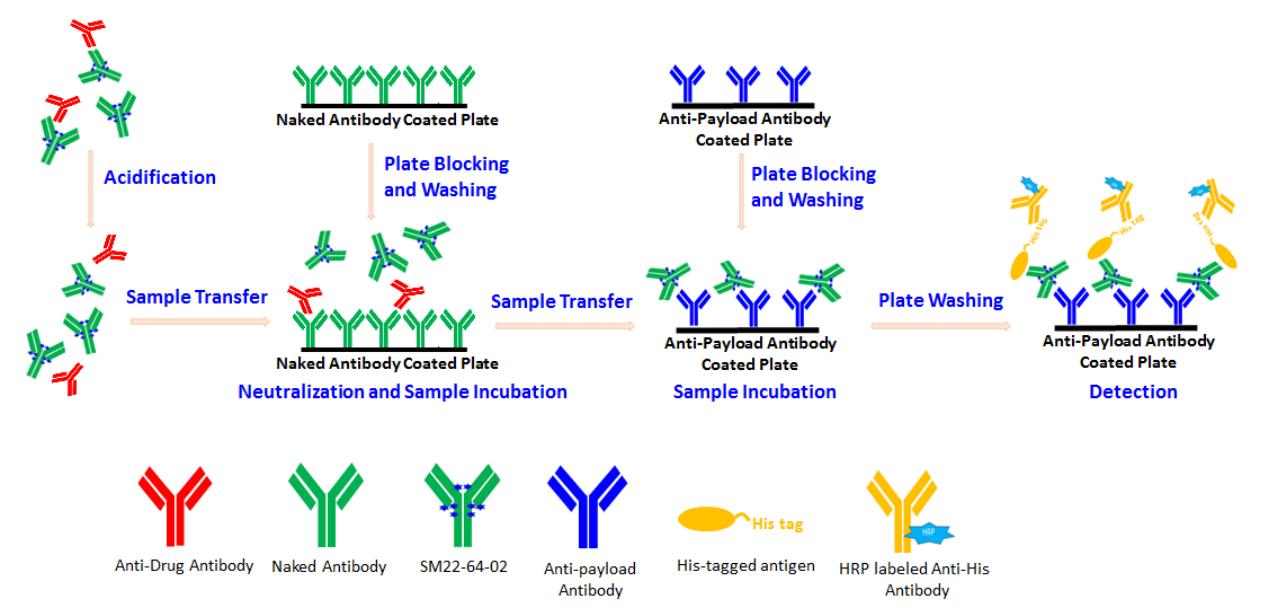 Fig.1 Schematic diagram of the present ADA-tolerant ADC assay.1, 2
Fig.1 Schematic diagram of the present ADA-tolerant ADC assay.1, 2
Features of our Services
- Easy to operate and automate
- Short turn-around time
- Competitive price with the best quality
- High sensitivity for low antibody concentrations
Based on a wealth of experience and cutting-edge technology, Creative Biolabs is confident in providing ADA screening assays for worldwide customers in the most high-quality and cost-effective way. Prior to the evaluation of samples in antibody assays, our professional staffs will help you clearly define the cut-point for better interpretation of results. In addition to ADA screening assays, we also provide other ADA immunogenicity assessment assays. We are happy to make it accessible to all kinds of research and industrial customers. If you are interested in the service we provide, please feel free to contact us for more information.
References
- Tao, Yimin, et al. "Development and Validation of an ADA-Tolerant Assay for Quantification of an Exatecan-Based ADC in Monkey Plasma." Molecules29.3 (2024): 572.
- Under Open Access license CC BY 4.0, without modification.
For Research Use Only.
