Fc based Function Analysis & Characterization
The development and manufacture of therapeutic antibodies represent a significant segment of the biopharmaceutical industry and have resulted in substantial benefits to public health. Therapeutic antibodies rely on two types of functionalities to achieve clinical efficacy: (i) target-specific binding by the variable domain and (ii) the immune-mediated effector functions via interaction of the Fc (crystallizable fragment) domain with cell receptors and complement proteins.
Having been concentrated on antibody therapy development for years, Creative Biolabs offers a diverse service portfolio to support new innovator or biosimilar therapeutic antibody development, including both variable and Fc domain related function analysis. Especially, we provide cell-based functional assays for evaluation of antibody effector functions and/or Fc engineering services for antibody functional modulation.
Introduction to Antibody Fc Function
The Fc region is the tail region of an antibody that interacts with cell surface receptors called Fc receptors and some proteins of the complement system, thereby triggering complex downstream signaling events and activating the immune system. In this way, Fc can mediate different physiological effects of antibodies, including detection of opsonized particles, cell lysis, and leukocyte degranulation. For therapeutic antibodies, the Fc region can have an important role in their safety and efficacy. Consequently, during antibody development and manufacturing, the Fc functionality should be well-studied and controlled.
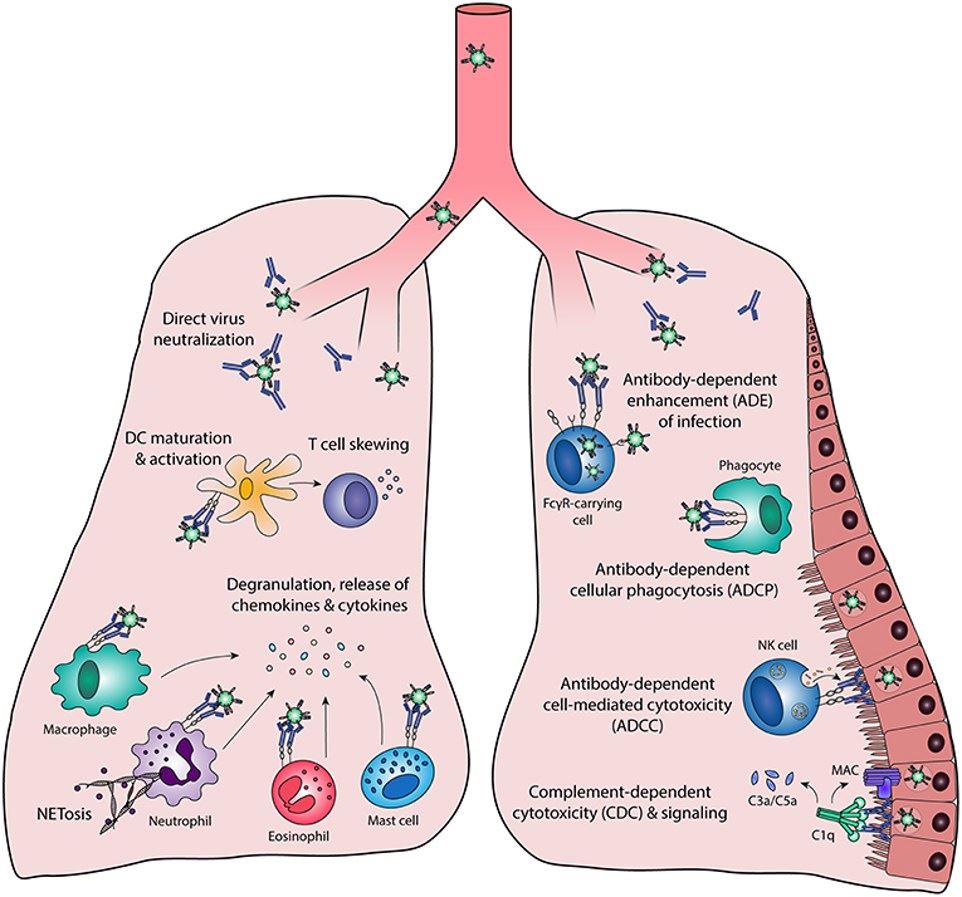 Fig.1 Functions of Fc-mediated antibody effectors.1, 2
Fig.1 Functions of Fc-mediated antibody effectors.1, 2
The assessment of effector functions is important for the development of original mAb candidates and biosimilar molecules. Leveraging Creative Biolabs' extensive expertise in Fc effector function assessment, we provide analysis and interpretation of the cytotoxic effects of your candidates in our highly reproducible antibody-dependent cellular cytotoxicity (ADCC), complement-dependent cytotoxicity (CDC), and antibody-dependent cell-mediated phagocytosis (ADCP) assays. Besides, to characterize your antibody product with respect to Fcγ receptor binding, we can also offer alternative non-cell-based assays, covering the whole Fc receptor panel and in addition FcRn. Furthermore, the binding affinity of antibody-antigen complexes to the complement component C1q can also be assessed with our advanced C1q binding assay platforms.
Although many antibody-based therapies have had clinical and commercial success, antibody-based therapeutics are often only effective in a subset of patients. Moreover, during development, many of the candidates have failed in the clinic due to poor efficacy. Therefore, in order to achieve greater efficacy, considerable effort has been put into fine-tuning the properties of antibodies. Among the many efforts, one area of focus is known as Fc engineering. At Creative Biolabs, we offer both antibody skeleton engineering, where the amino acid sequences are altered, and Fc glycoengineering, where the Fc glycosylation is modified, to optimize your antibody effector functions.
Service Advantages
- Versatile menu of assays
- Flexible technology platforms
- Broad scientific expertise
Frequently Asked Questions
FAQ
-
Q1: What biophysical advantages arise from Fc domain fusion in protein engineering?
A1: The Fc domain enhances fused proteins through structural robustness, improving thermodynamic stability and aqueous solubility—critical attributes for optimizing recombinant protein production and purification processes.
-
Q2: How do Fc-mediated mechanisms influence antiviral immunity beyond neutralization?
A2: Emerging evidence demonstrates that antibodies employ Fc-dependent effector functions to restrict pathogens via non-neutralizing pathways, a mechanism vital for mitigating viral evolution and enhancing the breadth of vaccine-elicited immunity.
-
Q3: What specimen categories are compatible with Fc functional characterization?
A3: Our services are adaptable for multiple sample types: (i) purified antibody preparations including monoclonal/polyclonal variants and Fc-containing fragments; (ii) complex biological specimens including cell culture supernatants, serum/plasma, and immune complexes.
-
Q4: What information is required for antibody functional profiling?
A4: Essential sample information includes molecular characteristics (e.g., mass specifications) and standardized nomenclature. Assay-specific parameters may necessitate additional descriptors, detailed in experimental workflows.
Creative Biolabs offers custom-developed functional assays to support your molecules at all stages of development. Our versatile menu of assays, flexible technology platforms, and broad scientific expertise provide a unique opportunity to evaluate the interaction of antibodies with Fc receptors, as well as to provide antibody Fc engineering strategies with the aim to further improve the performance of therapeutic antibodies. To find out more about how we could help with your research, contact us or directly sent us an inquiry.
References
- Van Erp, Elisabeth A., et al. "Fc-mediated antibody effector functions during respiratory syncytial virus infection and disease." Frontiers in Immunology 10 (2019): 548.
- under Open Access License CC BY 4.0, without modification.
For Research Use Only.
