Recombinant Adenovirus Rescue Service in Mammalian Cells
Recombinant adenoviruses (rAd) represent a cornerstone technology in biomedical research and therapeutic development. Their unparalleled transduction efficiency across a vast spectrum of dividing and non-dividing cells, high viral titer production, and capacity to accommodate large and complex genetic payloads make them an indispensable vector for diverse applications. These range from fundamental gene function analysis and in vivo disease modeling to the cutting-edge fields of oncolytic virotherapy and vaccine development. The critical gateway to leveraging these capabilities is the efficient and reliable rescue of the recombinant virus—the process of generating infectious viral particles from a DNA construct within a permissive mammalian cell line.
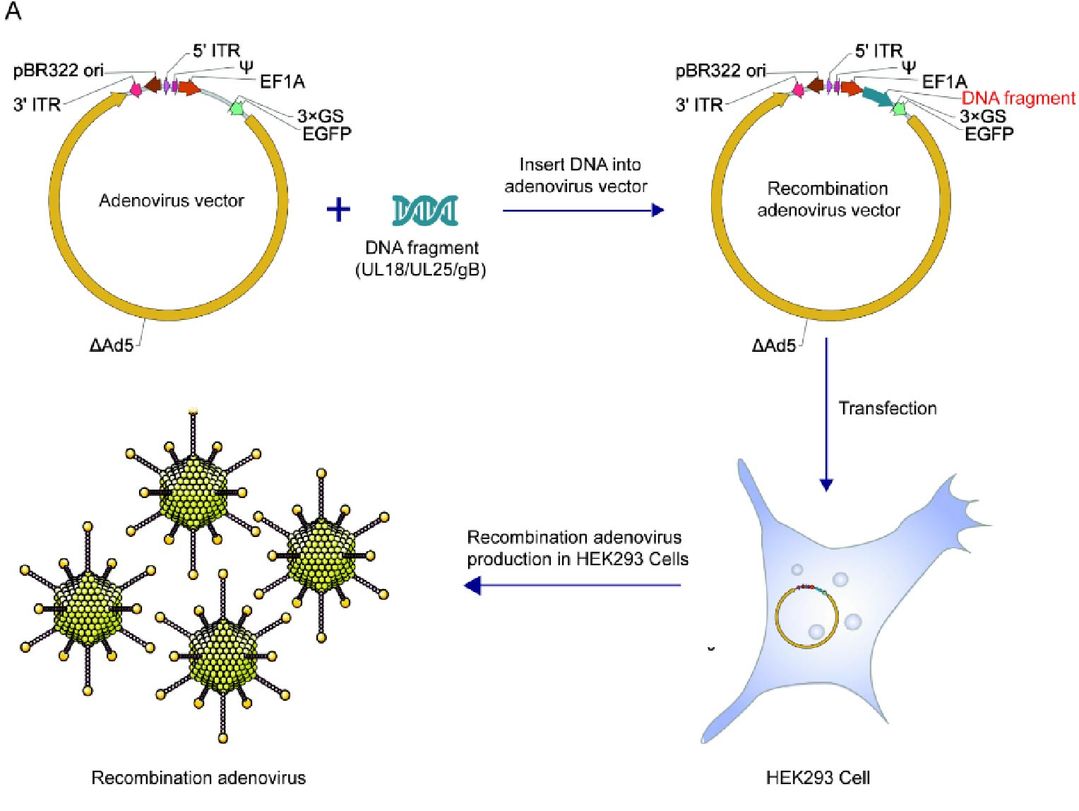 Fig.1 Constructing recombinant adenovirus for the immune response in mice 1,2
Fig.1 Constructing recombinant adenovirus for the immune response in mice 1,2
Our Recombinant Adenovirus Rescue Service in Mammalian Cells is meticulously designed to master this critical phase. We specialize in transforming your engineered adenoviral genome into a high-titer, pure, and functionally validated viral stock, empowering your research with robustness and reproducibility from the very start.
Our Comprehensive Adenovirus Rescue Workflow
We employ a systematic, phase-gated workflow that emphasizes transparency and rigorous quality control at every stage, ensuring the integrity and performance of your final viral product.
Project Initiation & Vector Integrity Check: The process begins with a thorough analysis and validation of your starting material. We verify the sequence and structural integrity of your adenoviral plasmid (e.g., based on AdEasy or similar systems), with a particular focus on the critical Inverted Terminal Repeats (ITRs) and your expression cassette, ensuring a solid foundation for rescue.
Linearization & Transfection: The adenoviral plasmid is precisely linearized, a crucial step that liberates the ITRs and initiates the viral genome replication process. This linearized DNA is then expertly transfected into our high-density, suspension-adapted HEK293 cell lines, engineered to provide the essential E1 genes in trans for replication-incompetent adenovirus production.
Plaque Assay & Clonal Isolation: To guarantee genetic purity and eliminate any non-recombinant background, we perform multiple rounds of plaque purification. This meticulous technique allows us to isolate a single, clonal viral plaque, which is then expanded. This step is fundamental to ensuring a homogeneous viral population, a prerequisite for consistent and interpretable experimental data.
Primary Amplification & Functional Validation: The clonal viral stock undergoes an initial round of amplification. The harvested lysate is then subjected to a suite of preliminary quality control tests. This includes PCR to confirm the presence of your transgene and the absence of wild-type contaminants, as well as functional assays to verify successful gene expression.
Large-Scale Production & Harvesting: Following successful validation, the virus is scaled up in controlled bioreactors to meet your required volume. We monitor the culture for the characteristic cytopathic effect (CPE), indicating successful viral replication, and harvest at the optimal time to maximize both yield and infectivity.
Concentration & High-Grade Purification: The crude viral harvest is concentrated and purified using industry-standard ultracentrifugation methods, such as Cesium Chloride (CsCl) density gradient centrifugation. This method is highly effective at separating full, infectious virions from empty capsids and cellular debris, resulting in a preparation of exceptional purity and a high infectious-to-genomic particle ratio.
Comprehensive QC & Final Formulation: The purified virus undergoes exhaustive characterization. We determine both the genomic titer (via qPCR/ddPCR) and the functional titer (via TCID₅₀ or plaque assay), and assess purity through SDS-PAGE. Stringent safety tests, including sterility, mycoplasma, and endotoxin analysis, are performed. The final product is formulated into a stable, ready-to-use buffer.
Delivery & Reporting: You receive your high-titer rAd stock, accompanied by a detailed Certificate of Analysis (CoA) that provides a complete quality snapshot, and a comprehensive project report documenting the entire journey from plasmid to purified virus.
Core Service Highlights
| Service Phase | Key Activities & Distinctive Advantages |
|---|---|
| Rescue & Clonal Isolation | Mandatory Multi-Round Plaque Purification: Guarantees a genetically homogenous viral population, crucial for data reproducibility. |
| Scalable Production | High-Yield Suspension Culture: Utilizes proprietary HEK293-derived cells in bioreactors for superior titers and scalability, from research to pre-clinical volumes. |
| Advanced Purification | CsCl Ultracentrifugation: Our standard method ensures superior separation of full vs. empty capsids, enhancing specific infectivity and in vivo performance. |
| Rigorous Quality Control | Multi-Parameter Analytics: Goes beyond titering to include functional potency, protein purity, and comprehensive safety profiling (RCA testing included). |
Our Distinct Advantages
Unmatched Expertise in Complex Constructs: We possess a proven track record in rescuing adenoviruses carrying large, complex, or unstable transgenes that often challenge standard systems. This makes us the preferred partner for projects involving multi-gene expression cassettes, CRISPR-Cas9 systems, and toxic genes.
Guarantee of Genetic Homogeneity: Our unwavering commitment to mandatory plaque purification sets us apart. This rigorous process eliminates the risk of working with a mixed viral population, providing you with the confidence that your experimental results are driven by a single, defined genetic entity.
Optimized Production Platform: Our proprietary, suspension-adapted HEK293 cell lines are engineered for high transfection efficiency and viral yield, while minimizing the generation of Replication-Competent Adenoviruses (RCA). This platform ensures both the safety and the cost-effectiveness of our production process.
Focus on Infectious Titer and Purity: We understand that the functional titer is what matters in your experiments. Our purification and QC strategy is specifically designed to maximize the infectious-to-genomic particle ratio, delivering a potent vector that performs reliably in vitro and in vivo, with minimal non-infectious background.
A Collaborative Scientific Partnership: We view ourselves as an extension of your team. Our scientists provide insightful consultation on vector design, promoter selection, and production strategies, leveraging deep virological expertise to help you avoid common pitfalls and accelerate your project timeline.
What Our Clients Say
"Our work required a panel of adenoviruses expressing different isoforms of a receptor, and some constructs were notoriously difficult to rescue. The team's systematic approach and expertise in handling problematic sequences were invaluable. They successfully delivered every single virus, all with exceptionally high functional titers. The detailed QC data, including the SDS-PAGE confirming capsid protein ratios, was a level of detail we don't see elsewhere."
— Dr. Anna Petrova, Group Leader
"Developing an oncolytic adenovirus presented unique manufacturing challenges. We chose this service for their emphasis on clonal purity and RCA testing. The result was a pristine viral stock that demonstrated potent anti-tumor efficacy in our models. Their scientists were true thought partners, providing critical feedback on the purification strategy that directly contributed to our success."
— Michael Reynolds, PhD, Co-Founder & CTO
"We needed a high-capacity adenovirus to deliver a whole metabolic pathway for a gene therapy proof-of-concept. The entire rescue and production process was seamless. The project management was proactive, and the final product exceeded our expectations in both titer and transduction efficiency in primary cells. The comprehensive CoA made dosing our animals straightforward and accurate."
— Dr. Jennifer Li, Principal Investigator
FAQ
What is the fundamental difference between adenovirus rescue in mammalian cells and AAV production?
The key difference lies in the viral life cycle and production complexity. Adenoviruses replicate and assemble their complex DNA-protein core within the nucleus of the host cell. Therefore, rescue must occur in mammalian packaging cells (like HEK293) that provide essential viral genes. This process involves active viral replication, leading to high yields. AAV production, conversely, involves co-transfecting cells with plasmids to assemble pre-formed capsids that are then packaged with the genome; the virus does not replicate, and the entire process is a single-round event.
Why do you insist on plaque purification, and can it be skipped?
Plaque purification is non-negotiable for rigorous science. Transfection can generate a heterogeneous mixture of recombinant, non-recombinant, and defective viruses. Skipping this step means your stock is a "soup" of different entities, leading to highly variable and irreproducible data. Our commitment to clonal purity is a commitment to the integrity of your research outcomes.
What starting materials are required to initiate a project?
We offer flexibility. The most efficient path is to provide a single, linearized adenoviral plasmid (e.g., pAdEasy-1 derived) where your expression cassette is already cloned and flanked by ITRs. We can also work with co-transfection of separate backbone and shuttle plasmids. We require a sufficient quantity of high-purity, sequence-verified plasmid along with the complete sequence map.
How is the safety of the final product ensured, particularly regarding RCA?
RCA (Replication-Competent Adenovirus) testing is a critical release criterion. We employ highly sensitive PCR-based assays specifically designed to detect the E1 gene sequence, which should be absent from the recombinant viral genome. Our optimized cell lines are also specifically chosen for their low propensity to generate RCA through homologous recombination. Any batch that fails this stringent test is rejected.
What deliverables can I expect upon project completion?
The Ready-to-Use Virus: High-titer, purified recombinant adenovirus, aliquoted and shipped under appropriate conditions.
Certificate of Analysis (CoA): A detailed document specifying the genomic titer (vg/mL), functional titer (IFU/mL or PFU/mL), purity data (SDS-PAGE), endotoxin level, and results of all safety tests (sterility, mycoplasma, RCA).
Comprehensive Project Report: A full narrative of the rescue, amplification, and purification process, providing complete traceability.
Conclusion
The successful rescue of a recombinant adenovirus is a pivotal and technically demanding step that can define the success of your entire research program. It requires more than just standard protocols; it demands deep virological insight, meticulous execution, and an unwavering commitment to quality. Our service is built upon this foundation, offering you not just a viral vector, but a reliable, potent, and thoroughly characterized research tool. By partnering with us, you gain access to a platform engineered for success, ensuring that your recombinant adenovirus is a robust driver of discovery, not a source of experimental variability. Let us handle the complexities of viral rescue, so you can focus on what you do best: pioneering scientific innovation.
References
- Zhang, H.; et al. Immune Response Elicited by Recombinant Adenovirus-Delivered Glycoprotein B and Nucleocapsid Protein UL18 and UL25 of HSV-1 in Mice. Int. J. Mol. Sci. 2024, 25, 13486.https://doi.org/10.3390/ijms252413486
- Distributed under Open Access license CC BY 4.0, without modification.
