UPF1 and Associated Diseases
Abnormal transcription of genetic information can lead to serious damage to cell function or genetics, and up-frameshift protein (UPF1) is an important part of the mRNA surveillance process to avoid this situation.
UPF1 and NMD
In eukaryotic cells, mRNA detection identifies aberrant transcripts and selectively degrades them through the process of nonsense-mediated mRNA decay (NMD). This process also removes misfolded peptides. Detection of mRNA and clearance of transcripts containing premature stop codons (PTCs) are critical for normal physiological function and survival of cells. The vast majority of eukaryotic cells achieve mRNA detection and clearance of PTC-containing transcripts through the ATPase and RNA helicase expressed by UPF1. Indeed, UPF1 is regulated by the upstream molecule SMG1. Mechanistically, UPF1 regulates NMD degradation through a phosphorylation/dephosphorylation cycle. UPF1 forms a complex with peptide release factors eRF1/eRF3 and is responsible for recognizing and terminating aberrant translation events in cells. Subsequently, the CH domain of UPF1 specifically binds to UPF2/3, releases the RNA helicase activity of UPF1 and functionalize the ATPase domain, and completely eliminates the abnormally expressed mRNA in cells.
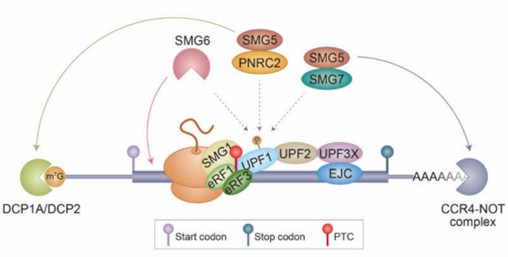 Fig 1. A model of NMD. (Hwang, 2021)
Fig 1. A model of NMD. (Hwang, 2021)
UPF1 and SMD
The degradation of mRNAs in cells that have a Staufen (STAU)1/2 binding site in their 3'UTR is achieved through Staufen-mediated mRNA decay (SMD). In fact, the STAU1 binding site (SBS) is a key factor in the SMD process. Essentially, the copolymer of STAU1 dimer and SBS will participate in the process of UPF1 recruiting UPF2, which will directly lead to the phosphorylation of UPF1 and the degradation of specific mRNA. Studies have confirmed the competition between SMD and NMD, which is caused by the fact that both STAU1 and NMD factors require the CH domain on UPF1 for specific binding.
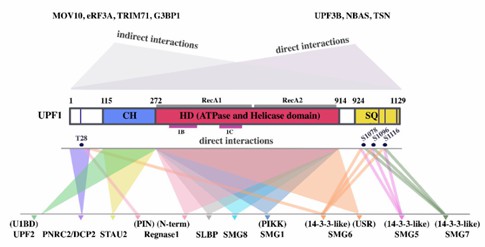 Fig 2. UPF1 domains and proteins that interact with them. (Lavysh, 2020)
Fig 2. UPF1 domains and proteins that interact with them. (Lavysh, 2020)
UPF1 in Cancer
The study reports that UPF1 is downregulated in most cell lines in the vast majority of cancers. Statistical analysis of clinical data shows that the disorder of UPF1 is directly related to the pathogenesis of liver cancer, gastric cancer, pancreatic cancer, prostate cancer, etc. and regulates the proliferation, invasion and migration of cancer cells. The overexpression of UPF1 can enhance the activity of Wnt pathway, induce the apoptosis of tumor cells, inhibit the expression of tumor stemness markers and significantly weaken the formation ability of tumor spheres. In contrast, down-regulation of UPF1 can increase tumor cell apoptosis and inhibit its proliferation. Studies have shown that UPF1 regulates epithelial-mesenchymal transition in response to TGF-β signaling, thereby controlling tumor metastasis. On the one hand, upregulated UPF1 directly inhibits the expression of TGF-β signaling genes MIXL1 and SOX17, on the other hand, overexpression of UPF1 can reduce the expression of Smad2 protein and inhibit TGF-β signal transduction. In addition, the interaction of UPF1 with long non-coding RNA (LncRNA) also regulate the expression and function of other genes and generate tumor phenotypes.
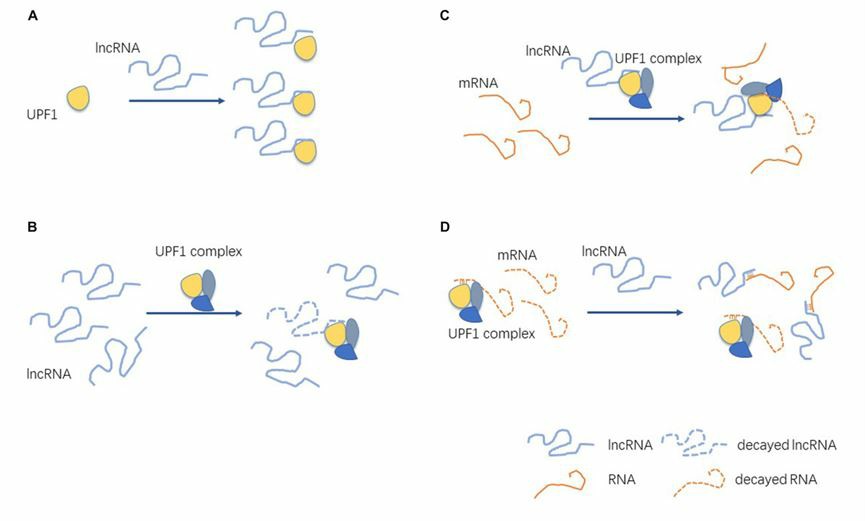 Fig 3. Interactions between UPF1 and LncRNA. (He, 2021)
Fig 3. Interactions between UPF1 and LncRNA. (He, 2021)
Creative Biolabs provides our clients with the most comprehensive one-stop UPF1 solution service. Our well-trained staff will assist you with experiments such as gene manipulation, sequencing, and provide you with the most reliable and reproducible research data. Please feel free to contact us.
References
- Hwang, H.J.; et al. UPF1: From mRNA surveillance to protein quality control. Biomedicines. 2021, 9: 995. Distributed under Open Access license CC BY 4.0, without modification.
- Lavysh, D.; et al. UPF1-mediated RNA decay—danse macabre in a cloud. Biomolecules. 2020, 10: 999. Distributed under Open Access license CC BY 4.0, without modification.
- He, J.; et al. Interactions between UPF1 and LncRNA in tumors. Frontiers in Genetics. 2021, 12: 624905. Distributed under Open Access license CC BY 4.0, without modification.
