Custom PEGylation Conjugation Services
Are you currently facing challenges in optimizing drug delivery, enhancing protein stability, or reducing immunogenicity? Creative Biolabs' Custom PEGylation Conjugation Service helps you overcome these obstacles and accelerate your biopharmaceutical development through advanced chemical modification techniques.
Introduction
PEGylation, the covalent attachment of hydrophilic and biocompatible polyethylene glycol (PEG) to therapeutic molecules like proteins and peptides, is a critical biopharmaceutical strategy. Achieved through diverse chemical reactions tailored to molecular functionalities and desired linkages, this modification yields significant enhancements. Notably, it improves aqueous solubility, a common limitation for many drug candidates, thereby optimizing pharmacokinetic profiles. Furthermore, PEGylation substantially increases molecular stability by sterically hindering enzymatic degradation and aggregation, leading to prolonged in vivo circulation and reduced dosing frequency. Critically, the PEG moiety diminishes immunogenicity by masking antigenic epitopes, allowing for repeated administration without eliciting strong immune responses. Since its inception in 1977, PEGylation has become indispensable in drug development, evidenced by numerous marketed and investigational PEGylated therapeutics that leverage these advantageous properties to improve efficacy and patient outcomes.
How Can Our PEGylation Service Assist Your Project?
Creative Biolabs provides tailored PEGylation solutions to enhance the therapeutic potential of your biomolecules. Our service delivers precisely engineered PEGylated conjugates with optimized properties, customized to your specific project needs. We offer comprehensive support, from initial consultation to final product delivery, ensuring seamless integration into your development pipeline.
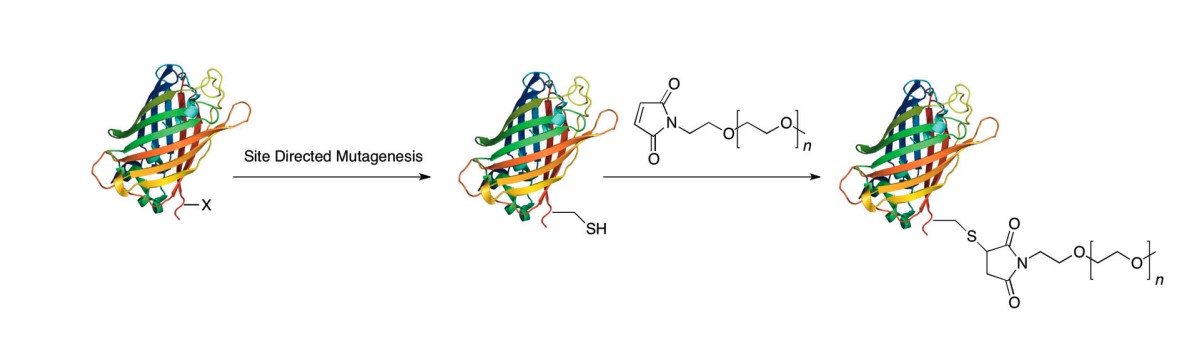 Fig.1 Site-specific PEGylation of a protein via chemical modification of cysteine.1
Fig.1 Site-specific PEGylation of a protein via chemical modification of cysteine.1
Our Service Types:
Creative Biolabs offers a comprehensive suite of PEGylation services, including:
- Protein-PEGylated Conjugation
- Peptide-PEGylated Conjugation
- Antibody-PEGylated Conjugation
- Structural analysis (optional methods such as mass spectrometry (MS), High Performance Liquid Chromatography (HPLC), dynamic light scattering (DLS) analysis)
Workflow:
Our streamlined workflow ensures efficient and reliable PEGylation, delivering high-quality products within a defined timeframe.
| Steps | Description |
| Initial Consultation and Project Design | We begin with a thorough consultation to understand your project goals, target molecule, and desired PEGylation outcome. Our team collaborates with you to design a customized PEGylation strategy, including selecting the appropriate PEG reagent, conjugation chemistry, and reaction conditions. |
| PEG Reagent Preparation and Activation | Creative Biolabs prepares and activates the chosen PEG reagent to ensure optimal reactivity and conjugation efficiency. This may involve functionalizing the PEG with specific reactive groups tailored to the target molecule's available conjugation sites. |
| Conjugation Reaction | The activated PEG reagent is then conjugated to your target molecule under carefully controlled conditions. We optimize reaction parameters such as temperature, pH, and stoichiometry to achieve the desired degree of PEGylation and minimize unwanted side reactions. |
| Purification and Characterization | Following the conjugation reaction, the PEGylated product is purified to remove unreacted PEG, byproducts, and other impurities. We employ various purification techniques, such as chromatography, filtration, and precipitation, to obtain a highly pure and homogeneous product. The purified conjugate is then characterized using a range of analytical methods, including MS, HPLC, and electrophoresis, to confirm its identity, purity, and PEGylation degree. |
| Quality Control and Final Delivery | Stringent quality assurance protocols are implemented to verify that the terminal PEGylated conjugate conforms precisely to client-defined specifications and quality benchmarks. We provide a comprehensive report detailing the synthesis, purification, and characterization of the conjugate, along with the final product. |
Required Starting Materials: To initiate a project, clients typically provide:
- Purified protein or peptide sample.
- Detailed information about the molecule's structure and properties.
- Specific requirements for the PEGylation, such as the desired PEGylation degree or site.
Final Deliverables:
- Purified and characterized PEGylated conjugate.
- Certificate of Analysis.
Estimated Timeframe:
The typical timeframe for this service ranges from 4 to 8 weeks, depending on the complexity and scope of the project, including the target molecule's properties, the desired PEGylation degree, and the required analytical characterization.
Our Advantages
- Cutting-edge Technological Expertise: Leveraging advanced PEGylation technologies to ensure highly efficient and precise modification processes, resulting in products with exceptional homogeneity.
- Experienced Team and Proven Expertise: Comprised of seasoned professionals with extensive theoretical knowledge and practical experience, providing clients with dependable technical support.
- Customized Conjugation Strategies: Offering a diverse array of conjugation methods and selectable modification sites to accommodate unique molecular characteristics and application requirements, enabling optimal functionalization.
- Comprehensive Quality Control System: Establishing a thorough PEGylated product characterization and analysis platform to guarantee that critical quality attributes, including modification efficiency, purity, and structure, meet stringent standards.
- Efficient Process Optimization Capabilities: Dedicated to streamlining PEGylation product manufacturing processes, assisting clients in achieving scalable production and enhanced cost-effectiveness.
Customer Reviews
FAQs
1. Q: What are the primary benefits of PEGylation?
A: PEGylation enhances drug solubility, stability, and circulation time while reducing immunogenicity. These improvements can lead to more effective and safer therapeutics. Contact us to discuss your specific molecule and how PEGylation can optimize its properties.
2. Q: How do I determine the optimal PEGylation strategy for my molecule?
A: Determining the optimal PEGylation strategy necessitates a comprehensive assessment of the molecule's inherent attributes and the intended functional consequences. Key determinants requiring rigorous evaluation include the selected PEG reagent, the conjugation chemistry employed, and the prevailing reaction parameters. Specialists at Creative Biolabs offer consultative services to facilitate the design of bespoke PEGylation strategies.
3. Q: What quality control measures are in place to ensure the quality of PEGylated products?
A: Creative Biolabs employs rigorous quality control procedures, including advanced analytical techniques, to ensure the identity, purity, and PEGylation degree of your product. We provide a comprehensive report and Certificate of Analysis with every delivery. Reach out to learn more about our quality assurance process.
4. Q: Can Creative Biolabs handle large-scale PEGylation projects?
A: Yes, Creative Biolabs maintains the operational capacity and specialized knowledge to manage PEGylation projects across a spectrum of magnitudes, encompassing both small-volume investigative studies and extensive industrial-scale production. Clients are encouraged to contact us to detail their project specifications and manufacturing demands.
5. Q: How does PEGylation compare to other drug modification techniques?
A: PEGylation offers unique advantages in terms of improving drug properties and reducing immunogenicity. While other techniques exist, PEGylation is often preferred for its biocompatibility and versatility. Let us help you evaluate if PEGylation is the right solution for your specific application.
How to Contact Us?
Creative Biolabs offers bespoke PEGylation conjugation services of exceptional quality, thereby facilitating advancements in biopharmaceutical development. Prospective clients are invited to engage our scientific team to delineate project specifications and explore collaborative avenues towards the realization of their research objectives. Contact Us Now!
Reference
- Dozier, Jonathan K., and Mark D. Distefano. "Site-specific PEGylation of therapeutic proteins." International journal of molecular sciences 16.10 (2015): 25831-25864. Under open access license CC BY 4.0, without modification.

