Peptide-Drug Conjugate (PDC) for Targeted Cancer Therapy
Background of Cancer Therapy
For decades, the therapeutic landscape of cancer has been dominated by conventional chemotherapeutics. These agents, however, are frequently characterized by their non-specific cytotoxicity, which results in significant systemic toxicity and a narrow therapeutic index. A central objective in modern oncological research, therefore, is the development of modalities capable of selectively delivering potent pharmacological agents to malignant cells, thereby minimizing damage to healthy tissues. Peptide-drug conjugates (PDCs) represent an emerging and highly versatile class of therapeutics designed to meet this objective.
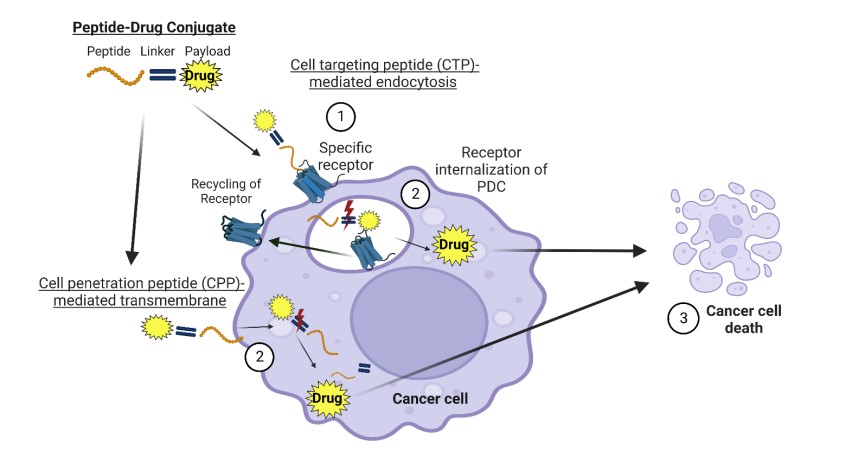 Fig.1 Receptor-mediated specific internalization of PDC in cancer cells.1
Fig.1 Receptor-mediated specific internalization of PDC in cancer cells.1
Key Components of PDCs
A peptide-drug conjugate is a tripartite molecular construct, the efficacy of which is contingent upon the synergistic function of its three constituent parts:
1. Peptide
This is the "GPS" of the PDC. Peptides, which are short chains of amino acids, serve as the targeting moiety. Their versatility allows for several advanced strategies:
- Tumor-Targeting Peptides (TTPs): The most common type, these are designed to bind with high specificity to receptors (like LHRH, EphA2, or SST2) that are overexpressed on the surface of cancer cells.
- Cell-Penetrating Peptides (CPPs): Rich in positive charges, these peptides have the intrinsic ability to cross the cell membrane, providing a direct route for transporting payloads into the cell's interior and overcoming certain types of drug resistance.
- Self-Assembling Peptides: These can form localized depots (supramolecular structures) within the tumor for sustained drug release.
- Brain-Penetrating Peptides: These are specifically designed to traverse the blood-brain barrier for central nervous system applications, such as Angiopep-2.
2. Linker
The linker, a chemical bridge between the peptide and payload, is engineered for systemic stability and conditional payload release at the target. Its technologies fall into two main categories:
- Cleavable Linkers: These can respond to specific physiological cues in the tumor microenvironment (e.g., pH, redox potential, or enzymatic activity)
- Non-Cleavable Linkers: These offer greater stability in plasma and rely on the complete degradation of the PDC within the lysosome of the cancer cell to release the active drug.
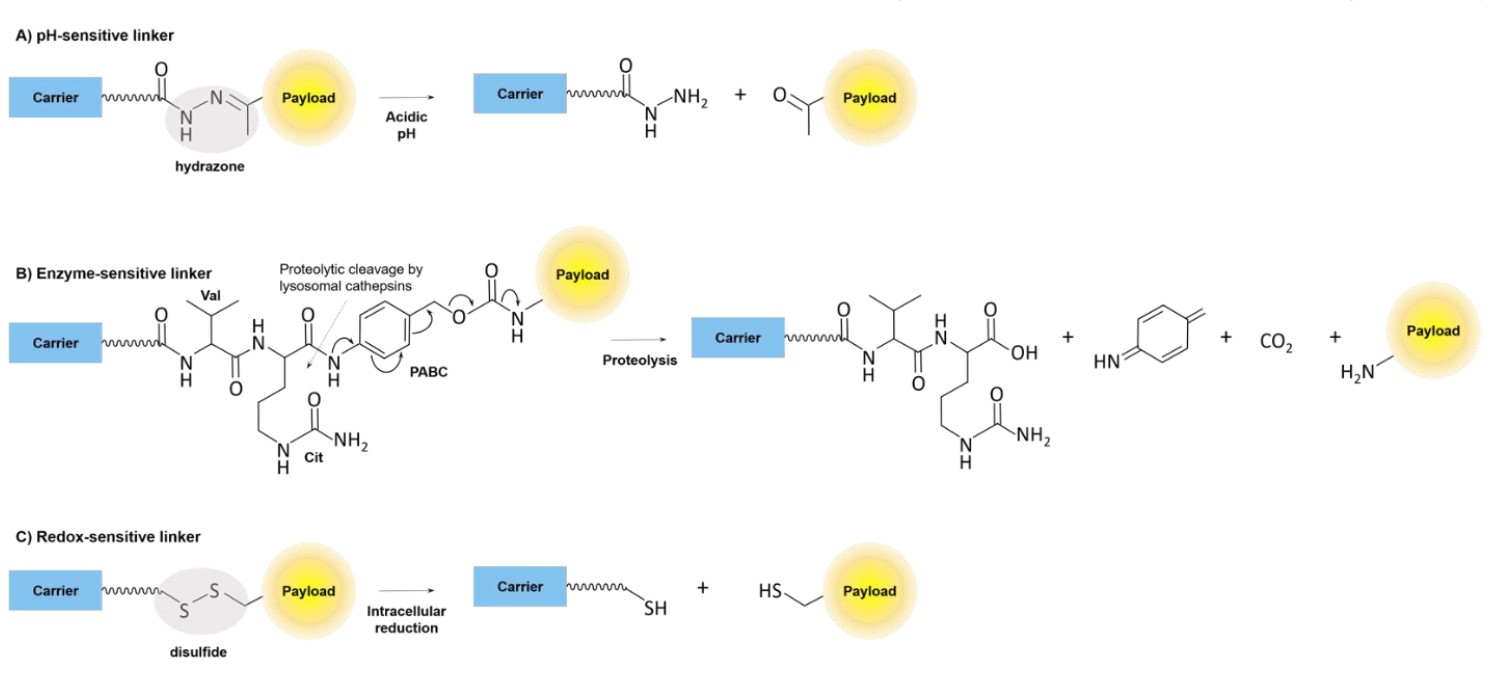 Fig.2 Cleavable linkers.1
Fig.2 Cleavable linkers.1
3. Cytotoxic Payload
This is the pharmacological effector moiety. The PDC platform accommodates a diverse array of payloads beyond traditional chemotherapeutics, including radionuclides for peptide receptor radionuclide therapy (PRRT), small interfering RNAs (siRNAs) for gene silencing, and other bioactive peptides, such as lytic peptides that disrupt cell membrane integrity.
Advantages of PDCs
While antibody-drug conjugates (ADCs) have successfully validated the principle of targeted cytotoxic delivery, PDCs possess both scientific and economic advantages that position them as the next generation of targeted therapeutics.
| Parameter | PDCs | ADCs |
|---|---|---|
| Molecular Mass & Tissue Penetration | Low (~2–20 kDa), facilitating enhanced penetration into the dense stroma of solid tumors. | High (~150 kDa), which can impede diffusion and limit therapeutic access to the tumor core. |
| Immunogenicity | Generally low, minimizing the potential for host immune responses. | Can elicit the formation of anti-drug antibodies (ADAs), potentially compromising efficacy and safety. |
| Synthesis & Cost-Effectiveness | Amenable to facile, scalable, and cost-effective solid-phase chemical synthesis. | Requires complex and costly biological manufacturing processes, including cell culture and protein purification. |
| Homogeneity & Quality Control | High degree of homogeneity, enabling straightforward characterization via standard analytical methods (e.g., HPLC, MS). | Characterized by inherent heterogeneity (e.g., drug-to-antibody ratio, glycosylation patterns), necessitating complex and stringent quality control protocols. |
| Pharmacokinetics | Exhibit a shorter plasma half-life and rapid renal clearance, which may reduce cumulative off-target toxicities. | Possess a prolonged half-life, which, while enabling less frequent dosing, may also lead to sustained off-target toxicity. |
Innovation of PDCs
While promising, PDCs face challenges that researchers are actively working to overcome, primarily related to their pharmacokinetic properties and delivery.
- Enhancing Stability and Pharmacokinetics: The small size of PDCs, while advantageous for tumor penetration, also leads to poor stability and rapid clearance by the kidneys. To address this, a host of innovative strategies are being developed, including PEGylation and the use of novel polymer technologies like polysarcosine to extend the half-life of PDCs in the bloodstream.
- The Rise of Advanced Architectures: The future of PDCs lies in even more sophisticated designs. Bicyclic toxin conjugates (BTCs), with their constrained structures, offer enhanced stability and target affinity. Peptide dendrimer conjugates (with their highly branched, multilayered architecture) allow for high drug loading and unique encapsulation capabilities.
- Enabling Oral Delivery: Currently, PDCs must be administered intravenously. The next great leap will be oral delivery. Nanotechnology approaches, such as using acid-stable coatings or anionic nanoparticles to enhance intestinal permeability, are being explored to make this a reality.
Creative Biolabs: Your Partner in Targeted Cancer Therapy
The development of a successful PDC is a complex, multidisciplinary undertaking, requiring deep expertise in peptide chemistry, advanced linker technology, and bioconjugation. At Creative Biolabs, we have the scientific knowledge and the state-of-the-art capabilities to help you navigate this exciting new frontier.
Our comprehensive suite of services includes:
- Custom Peptide Synthesis: We can design and synthesize high-purity TTPs, CPPs, and other advanced peptide architectures with the precise targeting properties you need.
- Small-Molecule Cytotoxic Payload Synthesis: Our chemists can synthesize a diverse portfolio of potent cytotoxic agents, from established chemotherapeutics to novel compounds, tailored to your specific therapeutic strategy.
- Expert Conjugation Services: Our scientists have extensive experience in conjugating peptides to a variety of payloads, ensuring the creation of a stable and effective PDC.
- Comprehensive Analytical Support: We provide a full range of analytical services to characterize your PDC and ensure its quality, purity, and performance.
Contact us today to learn how Creative Biolabs can accelerate your PDC program!
Reference
- Heh, Ethan, et al. "Peptide drug conjugates and their role in cancer therapy." International Journal of Molecular Sciences 24.1 (2023): 829. Under open access license CC BY 4.0, without modification.
