Precision Nanomedicine for Non-Small Cell Lung Cancer (NSCLC) Therapy
The Challenge of NSCLC Treatment
Non-small cell lung cancer (NSCLC) accounts for the vast majority (up to 85%) of pulmonary malignancies and represents a significant global health burden, which remains a leading cause of cancer-related deaths worldwide. A major impediment to successful therapeutic intervention is the typically indolent progression of the disease, which often results in diagnosis only at advanced, metastatic stages. For an extended period, the therapeutic paradigm has been anchored in a multimodal approach integrating surgical resection, radiotherapy, and cytotoxic chemotherapy. However, these established modalities, despite their utility, are fundamentally non-specific. Their mechanism of action frequently induces substantial collateral damage to non-malignant tissues and precipitates severe adverse events. Compounding these challenges is the profound intra- and inter-tumoral heterogeneity inherent to NSCLC. This genetic diversity ultimately facilitates the emergence of acquired resistance to such uniform therapeutic pressures, a phenomenon that culminates in unfavorable long-term survival statistics for a large patient population. Consequently, this clinical reality has created an urgent, unmet need for more intelligent, targeted, and personalized therapeutic strategies.
Biomarkers for NSCLC
Precision medicine is revolutionizing oncology, particularly in NSCLC. NSCLC comprises distinct subtypes characterized by actionable driver mutations. Identifying these key molecular biomarkers is paramount for effective targeted therapy design. Validated targets for these therapies include:
- EGFR (Epidermal Growth Factor Receptor): Activating mutations in this receptor create a clear target for EGFR inhibitors like gefitinib, which is designed to shut down its signaling.
- ALK (Anaplastic Lymphoma Kinase): Rearrangements involving the ALK gene produce an oncogenic fusion protein that can be selectively inhibited by drugs such as ALK inhibitors.
- KRAS (Kirsten Rat Sarcoma Virus): Previously considered undruggable, specific mutations like KRAS G12C are now targetable with novel covalent inhibitors that lock the protein in an inactive state.
- Other Critical Targets: A growing list of other mutations, including ROS-1, MET, BRAF, RET, HER-2, and NTRK, provides further opportunities for highly specific drug development.
This target-centric approach, guided by a tumor's unique genetic blueprint, allows for the design of drugs that precisely target and shut down the specific pathways fueling cancer growth, offering the promise of dramatically improved efficacy and safety.
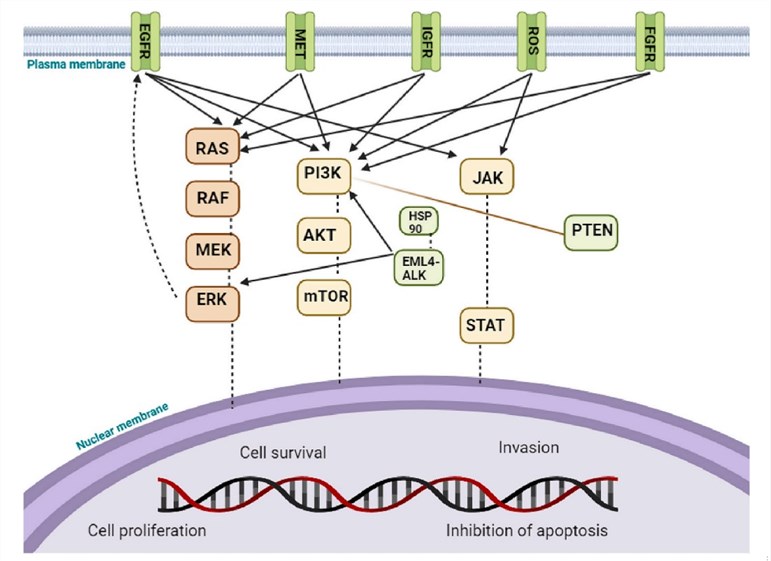 Fig.1 Molecular signaling pathways of NSCLC.1
Fig.1 Molecular signaling pathways of NSCLC.1
Nanoparticle Delivery Systems
Identifying the right target is only half the battle; delivering the therapeutic payload effectively is the other. This is where precision nanomedicine comes into play. Nanoparticles (NPs) are sophisticated, sub-micron carriers engineered to encapsulate potent anticancer agents and deliver them directly to the tumor microenvironment. This approach offers transformative advantages:
- Enhanced Specificity: NPs can be "functionalized" with targeting ligands (e.g. antibodies, targeted inhibitors, or aptamers) to selectively bind to overexpressed receptors on cancer cells. This strategy ensures preferential drug accumulation at the tumor site, enhancing therapeutic efficacy while minimizing off-target effects.
- Reduced Systemic Toxicity: By protecting the encapsulated drug from circulating in the bloodstream and sparing healthy tissues, NPs significantly widen the therapeutic window and minimize side effects.
- Improved Bioavailability: NPs protect the drug from premature degradation and clearance, increasing its circulation time and ensuring a higher effective dose reaches the tumor.
Key NP platforms leading the charge in NSCLC research include versatile liposomes, self-assembling polymeric micelles, and highly functional gold nanoparticles (AuNPs), each offering unique properties for custom drug delivery solutions.
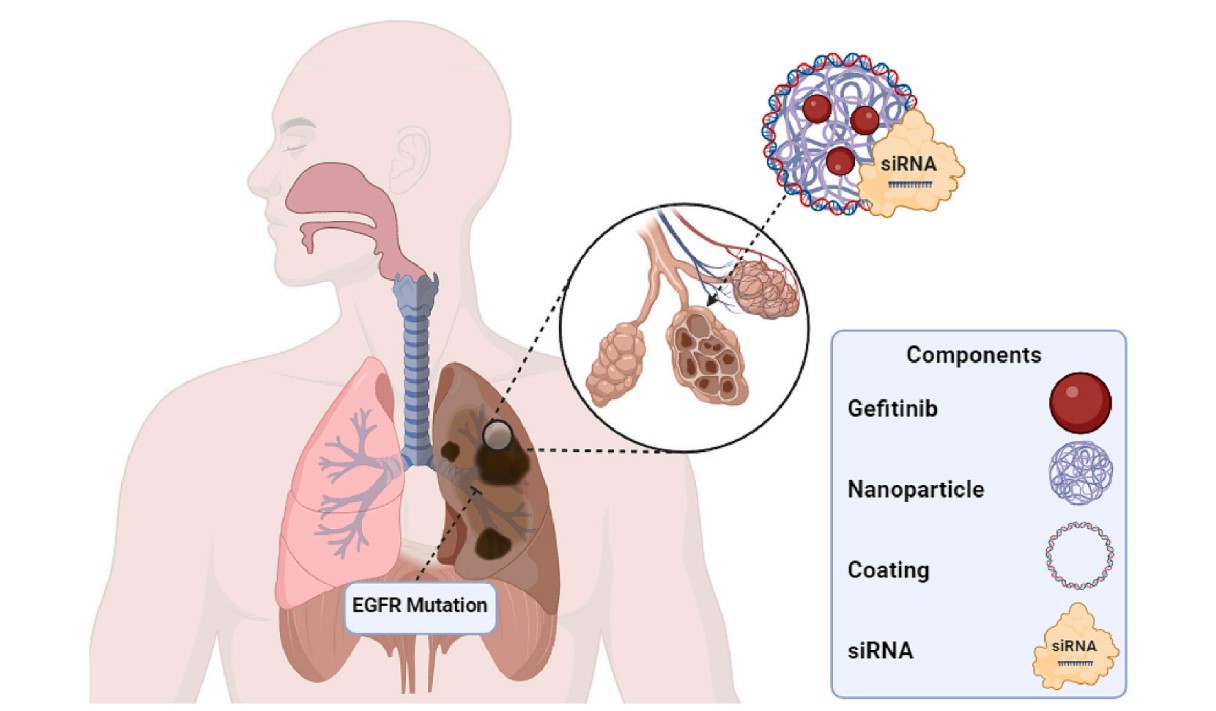 Fig.2 Nanoparticle strategies for targeted delivery in NSCLC.1
Fig.2 Nanoparticle strategies for targeted delivery in NSCLC.1
Your Partner for Targeted NSCLC Therapy
The convergence of biomarker-driven drug design and advanced nanodelivery systems holds immense potential to transform outcomes for NSCLC patients. However, navigating the complexities of formulation, targeting, and preclinical validation requires deep expertise and specialized capabilities. Creative Biolabs is your dedicated partner in this journey. We provide a comprehensive suite of services designed to minimize your development risks and accelerate your project schedule.
Our Core Service Capabilities:
-
Targeted Liposome Customization
We specialize in the design and synthesis of custom liposomal formulations for encapsulating small molecules, peptides, and nucleic acids. Our service includes surface modification with antibodies, aptamers, or other ligands to ensure precise targeting of NSCLC biomarkers. -
Targeted Gold Nanoparticle (AuNP) Customization
We offer tailored AuNP synthesis, size control, and conjugation with your specific targeting moiety and therapeutic payload, creating powerful platforms for combined imaging and therapy. -
Comprehensive Analytical Support
We provide a full suite of analytical services to ensure your formulation is optimized and well-characterized, including:- Particle size analysis (DLS) (basic)
- Zeta potential (basic)
- Encapsulation efficiency (basic)
- Drug loading (basic)
- Morphology (TEM) (optional)
- In vitro release kinetics (optional)
-
Preclinical In Vitro & In Vivo Validation
We offer a range of preclinical evaluation services, including cell line cytotoxicity assays on relevant NSCLC models, cellular uptake studies, and in vivo efficacy and biodistribution studies in xenograft models.
Contact us for more details.
Reference
- Dessai, Akanksha, Usha Yogendra Nayak, and Yogendra Nayak. "Precision nanomedicine to treat non-small cell lung cancer." Life Sciences (2024): 122614. Under open access license CC BY 4.0, without modification.
