Introduction How Can We Help? WorkflowDeliverables Case Study Why Choose Us? Published Data FAQ Related Services Featured Products
Are you currently facing challenges in evaluating complement‑activation risks, inconsistent anticomplementary activity assay (ACA) test results, or difficulties meeting regulatory safety requirements? Creative Biolabs' ACA Service helps you obtain reproducible, high‑confidence complement‑activation data through validated methodologies and standardized workflows powered by robust bioassay platforms.
Introduction of Anticomplementary Activity Assay (ACA)
The ACA assay measures unintended complement activation caused by immunoglobulin aggregates or therapeutic proteins. Complement activity can vary widely across laboratories without standardization, reinforcing ACA as a critical safety indicator for IVIG and biologics (e.g., aggregate‑induced complement activation, complement batch variability, and benefits of reference materials).
How Creative Biolabs' ACA Service Can Assist Your Project
Creative Biolabs provides a reliable, standardized ACA testing solution that quantifies complement consumption using highly controlled assay conditions. Clients gain clear, actionable insights into complement‑activation risks, product batch comparability, and regulatory readiness.
Discover How We Can Help – Request a Consultation.
ACA Assay Workflow
Applicable Sample Types
-
Plasma‑derived immunoglobulins (IVIG).
-
Monoclonal antibodies and Fc‑fusion proteins.
-
Recombinant protein therapeutics.
-
Novel biologic formats require complement safety assessment.
-
…
Sample Required
-
Test article information (formulation, concentration, storage conditions).
-
Minimum 0.5-1 mL of sample per condition.
-
Any reference comparators or control batches for comparability testing.
Key Steps Involved
01
Complement-Sample Incubation: Serum complement is incubated with the test material at 37°C to assess complement activation potential.
02
Erythrocyte Challenge Reaction: Sensitized erythrocytes are added to quantify residual complement via hemolysis intensity.
03
Reaction Termination: Stop solution is introduced, and samples are centrifuged to remove intact cells.
04
Assessment of Supernatant and Optical Density: Hemolysis intensity, indicative of complement consumption, is quantified at 415 nm.
05
Analysis and Interpretation of Data: Optical density readings are standardized against control samples, with ACA levels computed through validated formulas to ensure precision.
Estimated Timeframe
Typical assay completion ranges from 2-3 weeks, depending on sample number, complexity, and batch comparability requirements.
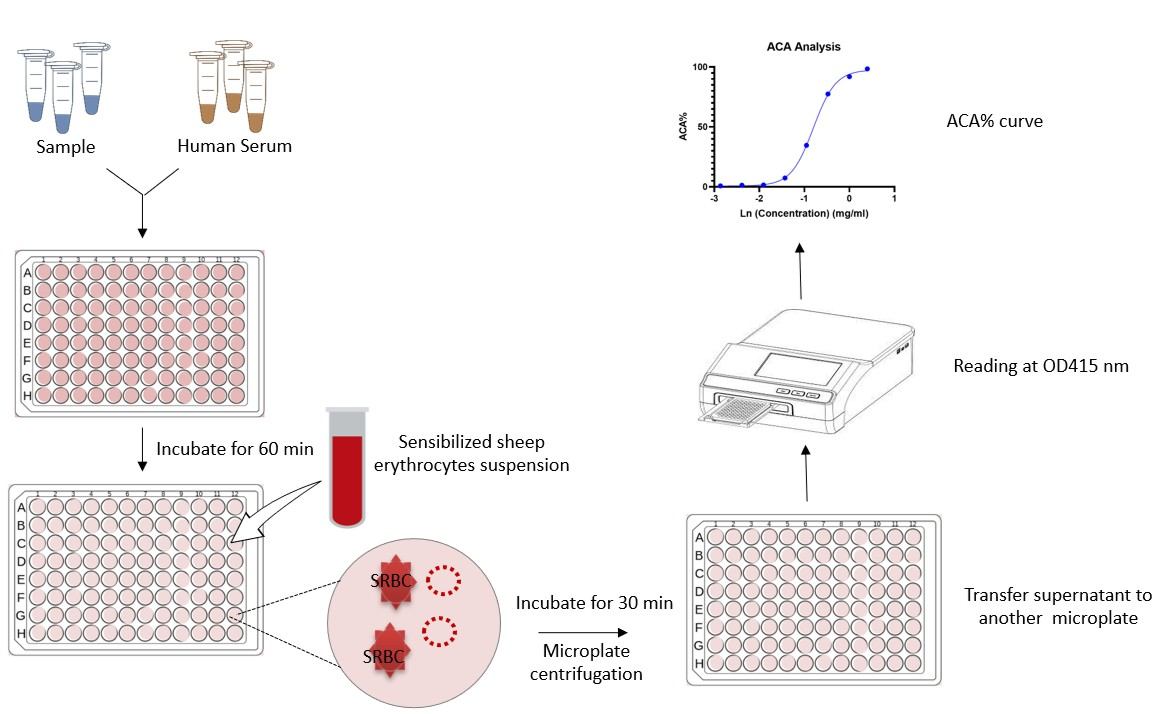 Fig.1 Schematic diagram of ACA assay procedure.
Fig.1 Schematic diagram of ACA assay procedure.
Deliverables
-
Quantified complement‑consumption dataset (OD based ACA values).
-
Fully documented experimental methodology.
-
Raw absorbance files and statistical outputs.
-
Batch comparability analysis (if applicable).
Case Study
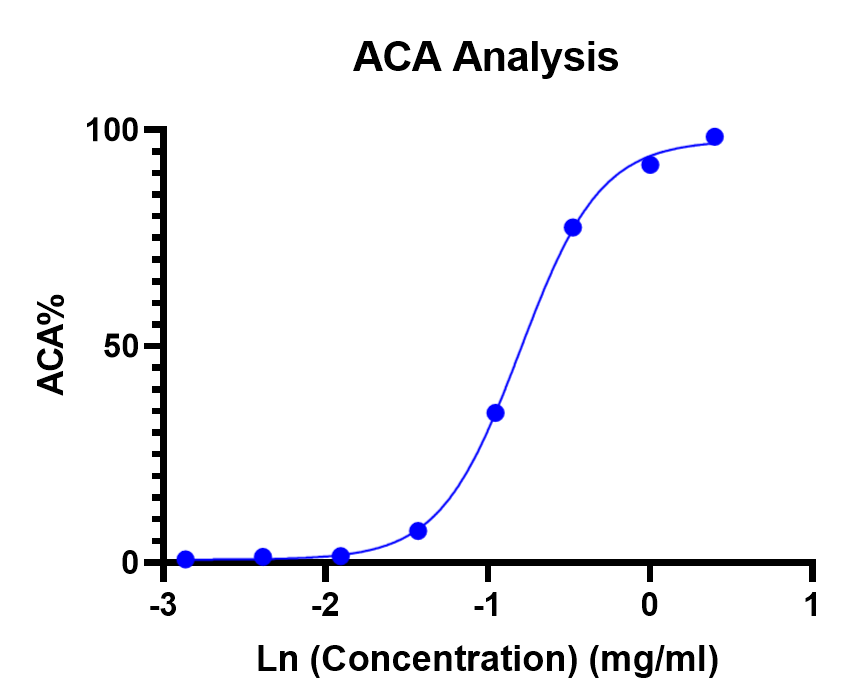 Fig.2 ACA assay test in the normal human serum.
Fig.2 ACA assay test in the normal human serum.
In this case, Creative Biolabs uses HAGG in human serum, the ACA assay reveals a clear, concentration-dependent, sigmoidal complement consumption: at low Ig concentrations, complement remains largely intact, but above a threshold concentration, nearly complete consumption occurs. This strongly implies that the HAGG preparation (or a sub-fraction within it) has substantial non-specific complement-activating capacity (likely due to aggregates or structurally altered IgG).
Why Choose Us?
Creative Biolabs offers a validated, one‑stop ACA testing solution designed for biologics developers seeking reliable complement‑activation analytics. Advantages include:
-
High reproducibility through standardized assay materials and protocols.
-
Rapid turnaround with clear, actionable interpretations.
-
Experienced scientific team with deep complement‑system expertise.
-
Flexible testing options for research, formulation screening, and regulatory support.
-
Published Data supporting the scientific rigor of ACA assays.
Discover the Creative Biolabs Edge – Request Your Quote Now!.
Published Data
Recent research underscores the crucial role of hemolysis-based ACA assays in evaluating complement activation by polymeric nanoparticles, emphasizing their utility in revealing unintended immune responses. The findings highlight how variations in particle size, surface charge, and chemical composition can profoundly influence complement consumption, facilitating enhanced material comparisons. ACA assays proved effective in identifying biomaterials with minimal complement activation, pivotal for advancing biocompatible therapeutic delivery systems. Additionally, the research identifies how manufacturing nuances can impact complement levels, accentuating ACA's relevance in optimizing production processes. Ultimately, ACA metrics are instrumental in guiding safer formulation selections and aligning with regulatory safety standards.
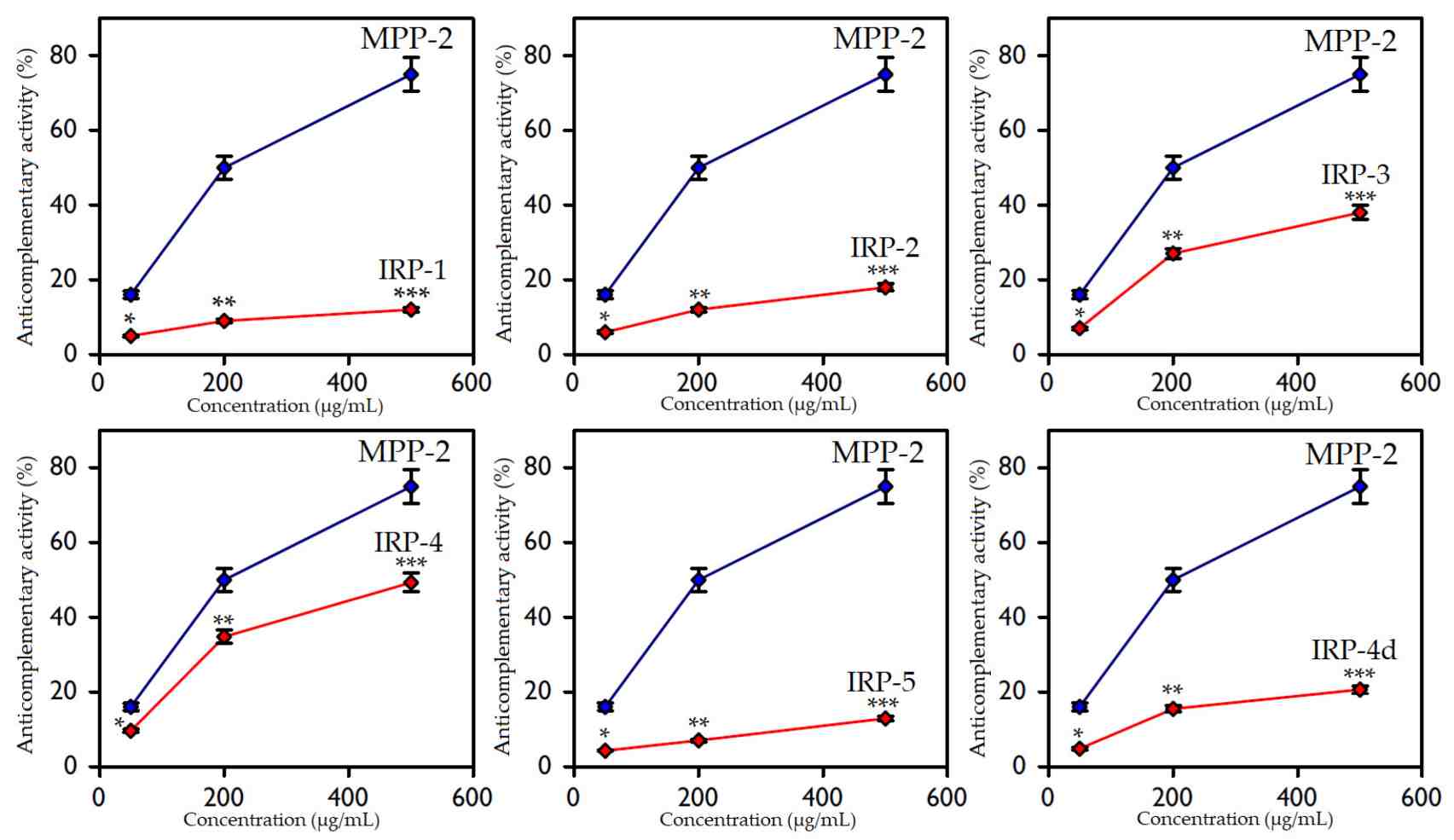 Fig.3 Assessment of complement inhibition by IRP-1–IRP-5 and IRP-4d polysaccharides.1
Fig.3 Assessment of complement inhibition by IRP-1–IRP-5 and IRP-4d polysaccharides.1
Frequently Asked Questions
Q1: What sample volume is required?
A: Typically 0.5-1 mL per condition. Contact us for customized requirements.
Q2: Can Creative Biolabs test multiple batches for comparability?
A: Yes, we routinely evaluate ACA across production lots.
Q3: How stable should my samples be before shipment?
A: We recommend cold‑chain storage; stability guidance is available upon request
Q4: Does ACA testing replace aggregate analysis?
A: No, ACA complements physicochemical analysis by evaluating biological activity.
Related Hot Products
Creative Biolabs also offers a range of complement testing services to further support your research on the complement system.
Featured Products
Creative Biolabs provides comprehensive Anticomplementary Activity Assay support, delivering accurate, reproducible complement‑activation data for biologics safety and development. Contact Our Team for More Information and to Discuss Your Project.
Reference
-
Olennikov, Daniil N., and Tatyana G. Gornostai. "New Inonotus polysaccharides: Characterization and anticomplementary activity of Inonotus rheades mycelium polymers." Polymers 15.5 (2023): 1257. Distributed under Open Access license CC BY 4.0, without modification. DOI: https://doi.org/10.3390/polym15051257
For Research Use Only.
Related Sections:

 Fig.1 Schematic diagram of ACA assay procedure.
Fig.1 Schematic diagram of ACA assay procedure.
 Fig.2 ACA assay test in the normal human serum.
Fig.2 ACA assay test in the normal human serum.
 Fig.3 Assessment of complement inhibition by IRP-1–IRP-5 and IRP-4d polysaccharides.1
Fig.3 Assessment of complement inhibition by IRP-1–IRP-5 and IRP-4d polysaccharides.1