Introduction Published Data What We Can Offer? Workflow Why Choose Us? FAQs Featured Services Featured Products
Accelerate Your Research and Development!
Are you currently facing challenges in developing new treatments for complement-mediated diseases or leveraging the complement system in your therapeutic strategy? Creative Biolabs' Complement System Therapeutic Drug Development Service for CD46 (MCP) helps you accelerate your drug discovery and obtain high-quality, targeted therapeutics through our advanced antibody engineering and high-throughput screening platforms.
Contact our team to get an inquiry now!
Introduction
The complement system is a crucial part of the innate immune response, acting as a rapid, cascade-like defense mechanism against pathogens. It also plays a vital role in clearing damaged cells and bridging innate and adaptive immunity. When this intricate system is dysregulated, it can lead to severe pathologies, including autoimmune diseases like atypical hemolytic uremic syndrome (aHUS) and systemic lupus erythematosus (SLE), and even contribute to neurodegenerative diseases and cancer.
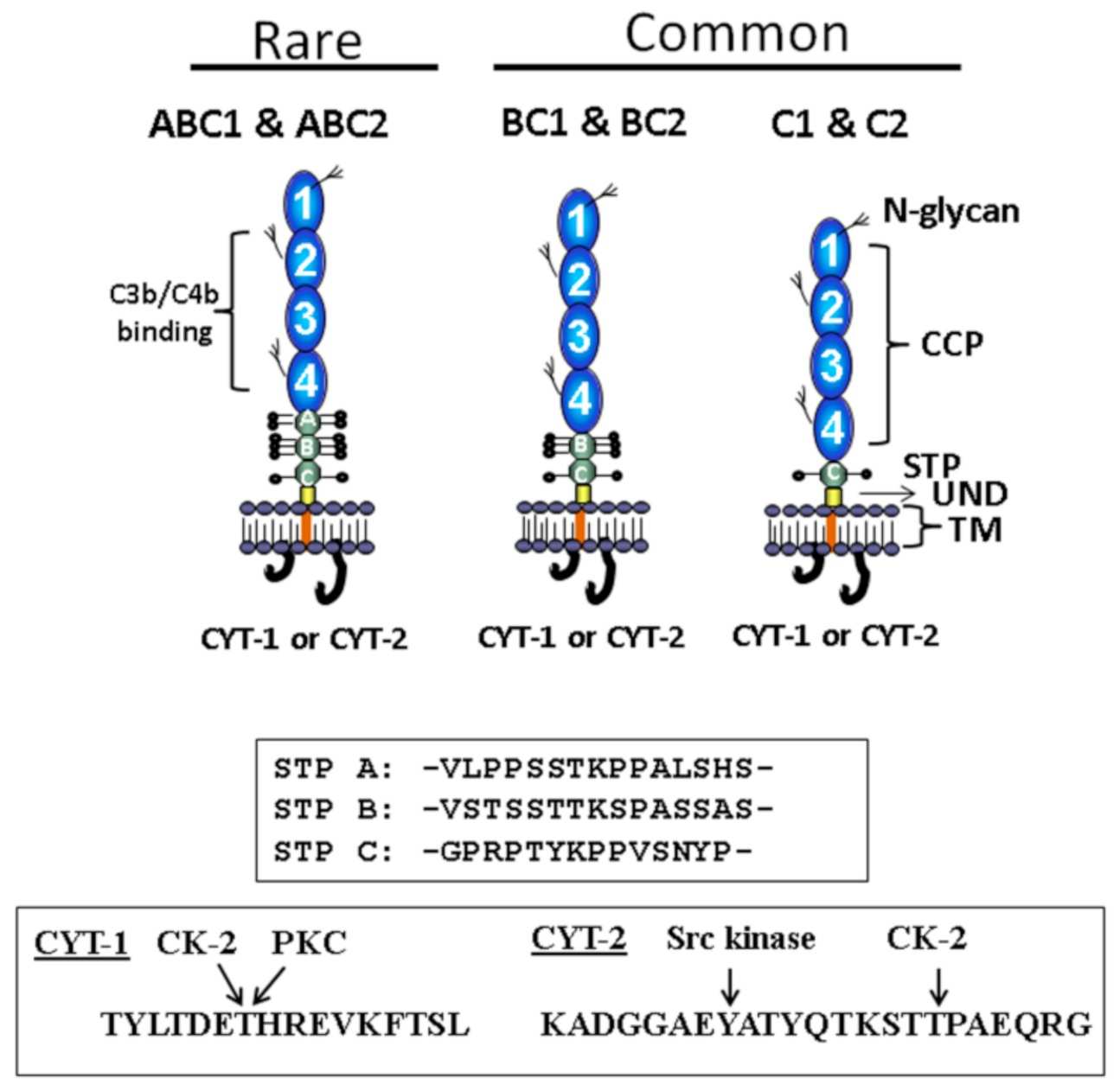 Fig.1 The structure of CD46.1,3
Fig.1 The structure of CD46.1,3
CD46, also known as Membrane Cofactor Protein (MCP), is a widely expressed, ancient molecule that acts as a key regulator of the complement cascade. CD46 functions as an essential cofactor for serine proteinase Factor I, permitting inactivation of complement cascade components C3b and C4b. This function is critical for protecting host cells from unintended complement attack. Research has shown that mutations in the CD46 gene are a predisposing factor for diseases like aHUS. Furthermore, CD46 is known to be overexpressed on many cancer cells, which use it as a protective shield to evade complement-mediated destruction. This dual role—as a protector of host cells and a shield for tumor cells—makes CD46 an exceptionally promising target for therapeutic intervention.
Targeting CD46 for Cancer Treatment
CD46 is highly expressed on the surface of many human cancers, including multiple myeloma, breast cancer, and glioblastoma. This overexpression is not accidental; cancer cells hijack the protein's native function to protect themselves from the complement system, a process known as complement evasion. The presence of high levels of CD46 allows tumors to withstand attacks from the innate immune system, making them resistant to both endogenous immune responses and certain immunotherapies.
Targeting CD46 in a therapeutic context aims to neutralize this protective mechanism. By developing agents that block or downregulate CD46, we can effectively "unmask" the tumor cell, making it susceptible to complement-mediated destruction. This approach can be particularly powerful when combined with other cancer therapies that rely on a functional immune response. Strategies range from using viral vectors that specifically target CD46-expressing cells to designing antibody-drug conjugates that deliver a cytotoxic payload directly to the tumor, all while bypassing the protective effect of CD46.
Complement Drugs Targeting CD46
The unique and multifaceted role of CD46 in both immunity and disease has made it an attractive target for therapeutic development. Unlike traditional complement inhibitors that block the cascade at a single point, CD46-targeting drugs are designed to specifically modulate the function of this key membrane protein.
Viral Vectors
Viral Vectors are a significant class of CD46-targeting therapeutics, especially in oncology. Certain adenoviral strains and measles viruses exploit CD46 as a cellular entry receptor. This mechanism has been exploited to engineer oncolytic viruses that selectively infect and destroy cancer cells that overexpress CD46.
Antibody-Drug Conjugates (ADCs)
Antibody-Drug Conjugates (ADCs) represent another innovative approach. A human monoclonal antibody that binds to CD46 can be used as a delivery vehicle to selectively transport a potent cytotoxic drug directly to cancer cells. This method targets the therapy to the tumor, minimizing off-target effects on healthy tissues.
CD46 Modulators
CD46 Modulators are a broad category of therapeutics being explored to either enhance or inhibit the protein's function. In scenarios like transplant rejection or autoimmune diseases, therapeutics that enhance CD46 activity can help dampen the complement response and protect host tissues. Conversely, in cancer immunotherapy, inhibiting CD46's function can make tumor cells more susceptible to complement-mediated attack.
Published Data
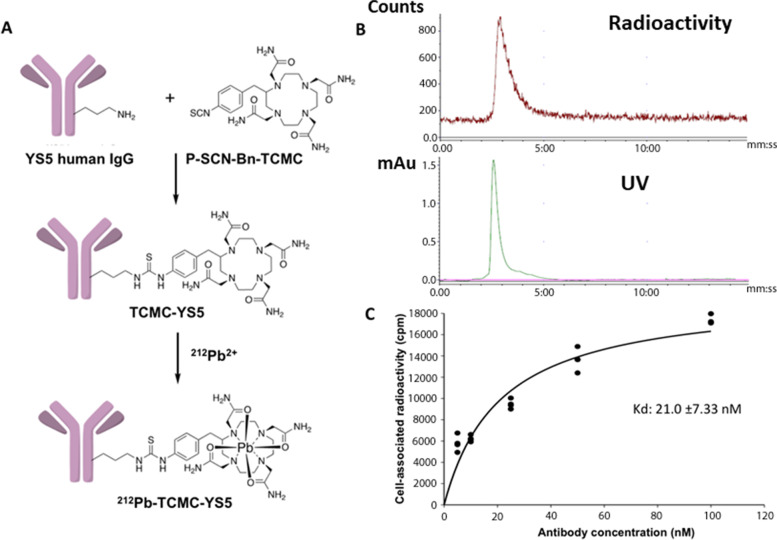 Fig.2 Preparation of 212Pb-TCMC-YS5 and cellular binding analysis in vitro.2,3
Fig.2 Preparation of 212Pb-TCMC-YS5 and cellular binding analysis in vitro.2,3
A recent study demonstrated the efficacy of a novel targeted alpha radioimmunotherapy, 212Pb-TCMC-YS5, for the treatment of metastatic castration-resistant prostate cancer (mCRPC). The therapy was designed to target the CD46 antigen, which was identified as being consistently expressed on both adenocarcinoma and small cell neuroendocrine subtypes of mCRPC. The researchers created a radioimmunoconjugate using the human monoclonal antibody YS5 and the alpha-emitting radionuclide 212Pb.
The experiment was conducted in three preclinical small animal models of prostate cancer: a subcutaneous xenograft model, an orthotopic xenograft model, and a patient-derived xenograft (PDX) model. A single administration of the therapy was shown to be well-tolerated and resulted in potent and sustained tumor inhibition across all models. The regimen markedly enhanced survival probabilities within the test cohort. These findings highlight CD46 as a viable and effective target for cancer therapy, showing a strong therapeutic window in preclinical models with potential for clinical translation.
What We Can Offer?
To support your research and development needs, we provide a range of products and services designed to help you succeed in the complement therapeutics space. Our offerings include:
-
Custom Antibody Development: Generation of monoclonal, polyclonal, and recombinant antibodies targeting CD46.
-
Protein Production and Purification: High-quality recombinant CD46 protein and other complement-related proteins for research and assay development.
-
Functional Assays: A full suite of complement activation and inhibition assays, including cell-based and ELISA-based methods to screen for active compounds.
-
Lentiviral Packaging Service: Production of high-titer lentivirus for stable gene expression and cell line engineering.
-
Cell Line Development: Generation of custom cell lines expressing or lacking CD46 for research and screening purposes.
Workflow
01
Target and Antigen Design: Our team begins by designing a specific antigen based on the CD46 protein, with an emphasis on key functional domains, such as the complement control protein (CCP) repeats.
02
Antibody Library Screening: Using our proprietary high-throughput platforms, we screen vast antibody libraries to identify a diverse range of binders with high affinity for the CD46 antigen.
03
Lead Identification and Optimization: Promising antibody candidates are rigorously evaluated for specificity, binding affinity, and their ability to modulate CD46 function in vitro (e.g., as a cofactor for Factor I-mediated cleavage of C3b/C4b). We then optimize the lead candidates to improve their therapeutic properties.
04
Functional Assays: The lead candidates are tested in a variety of functional assays to confirm their desired biological effect, such as protecting host cells from complement attack or, conversely, sensitizing cancer cells to complement-dependent cytotoxicity (CDC).
05
Final Development and Delivery: The optimized lead is prepared for final delivery, including comprehensive data packages and cell lines for future development.
Why Choose Us?
Creative Biolabs' expertise in developing therapeutics for CD46 is built on years of experience and a deep understanding of the complement system. We offer a unique combination of scientific acumen and an advanced technology platform to ensure project success.
-
Deep Scientific Expertise: Our team consists of seasoned biologists and immunologists with decades of experience in complement biology and therapeutic development.
-
End-to-End Service: We provide a comprehensive service from initial target design to final lead candidate delivery, streamlining your R&D process and reducing time and costs.
-
Cutting-Edge Technology: We leverage state-of-the-art platforms for high-throughput screening and functional validation to identify the most potent and specific therapeutic candidates.
-
Proven Track Record: Our published data and previous successes in complex therapeutic areas attest to our ability to deliver high-quality results. Our approach has led to the successful development of novel immunomodulators and targeted therapies.
-
Customized Solutions: We tailor our workflow to meet the specific needs of your project, whether you require an agonist, antagonist, or a simple binding molecule.
Obtain the Creative Biolabs Advantage – Seek a Quote Today
FAQs
Q: How can a CD46-targeting therapeutic be used to treat both autoimmune and cancer indications?
A: CD46 plays a dual role in the body. In autoimmune diseases like aHUS, where there's excessive complement activation, a therapeutic that enhances CD46's regulatory function could help protect healthy cells from attack. Conversely, in certain cancers where tumor cells overexpress CD46 to evade immune detection, a therapeutic that blocks or inhibits CD46 could make the tumor more vulnerable to the complement system, enhancing the efficacy of immunotherapies.
Q: What is the mechanistic difference between targeting a complement regulator like CD46 versus a downstream component like C5?
A: Targeting a complement regulator such as CD46 is an upstream approach. By modulating CD46's ability to inactivate C3b and C4b, you are influencing a key checkpoint in the cascade, which can affect both the classical and alternative pathways. This may offer a broader, more nuanced control over the entire system. In contrast, targeting a downstream component like C5 primarily blocks the final, common pathway of complement activation, specifically preventing the formation of the membrane attack complex (MAC). While highly effective for preventing lytic cell death, it does not address the upstream inflammatory and opsonization processes mediated by C3 and C4.
Q: How does the structure of CD46 influence its function as a complement regulator?
A: The structure of CD46 is key to its function. It consists of four short consensus repeats (SCRs), which are also known as complement control protein (CCP) repeats. These domains are responsible for binding to the complement proteins C3b and C4b. Mutations or modifications within these domains can disrupt this crucial binding, leading to dysregulation of complement and contributing to disease.
Q: How does CD46 function differently in normal versus malignant cells?
A: In a healthy cell, CD46 acts as a critical self-protective mechanism, ensuring that the complement system does not mistakenly attack host tissues. It serves as a passive shield. In many cancer cells, however, CD46 is highly overexpressed and actively exploited for survival. The cancer cell essentially weaponizes this protein to create a robust defensive barrier against immune-mediated destruction, making it a prime target for therapies designed to re-sensitize the tumor to immune attack.
Creative Biolabs' Complement System Therapeutic Service for CD46 (MCP) offers a comprehensive solution for developing innovative treatments that modulate the complement system. Our expertise, advanced technology, and tailored approach ensure we can help you accelerate your project and achieve your therapeutic goals.
Featured Services
Feature Products
References
-
Elvington, Michelle et al. "CD46 and Oncologic Interactions: Friendly Fire against Cancer." Antibodies (Basel, Switzerland) vol. 9,4 59. 2 Nov. 2020, https://doi.org/10.3390/antib9040059
-
Li, Jun et al. "CD46 targeted 212Pb alpha particle radioimmunotherapy for prostate cancer treatment." Journal of Experimental & Clinical Cancer Research: CR vol. 42,1 61. 11 Mar. 2023, https://doi.org/10.1186/s13046-023-02636-x
-
Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.
Related Sections:

 Fig.1 The structure of CD46.1,3
Fig.1 The structure of CD46.1,3
 Fig.2 Preparation of 212Pb-TCMC-YS5 and cellular binding analysis in vitro.2,3
Fig.2 Preparation of 212Pb-TCMC-YS5 and cellular binding analysis in vitro.2,3