Introduction Published Data What We Can Offer? Workflow Why Choose Us? FAQs Featured Services Featured Products
Accelerate Your Research and Development!
Are you facing challenges with developing new treatments for autoimmune, inflammatory, or oncology indications? The complexity of the complement system often presents a significant hurdle. Our Complement System Therapeutic: Drug Development Service for CD55 (DAF), powered by Creative Biolabs' innovative protein engineering techniques, helps you overcome these obstacles. We assist in accelerating drug discovery and obtaining high-quality therapeutic candidates by providing specialized solutions for modulating the complement cascade.
Contact our team to get an inquiry now!
Introduction
The complement system is a vital component of the innate immune response, acting as a powerful defense against pathogens. However, its uncontrolled activation can lead to significant tissue damage in various autoimmune, inflammatory, and neurodegenerative diseases. CD55, also known as Decay-Accelerating Factor (DAF), is a key regulator of this system. It is a glycosylphosphatidylinositol (GPI)-anchored cell surface protein that protects host cells from complement-mediated attack by accelerating the decay of C3 and C5 convertases. This crucial function prevents the formation of the Membrane Attack Complex (MAC), which would otherwise cause cell lysis.
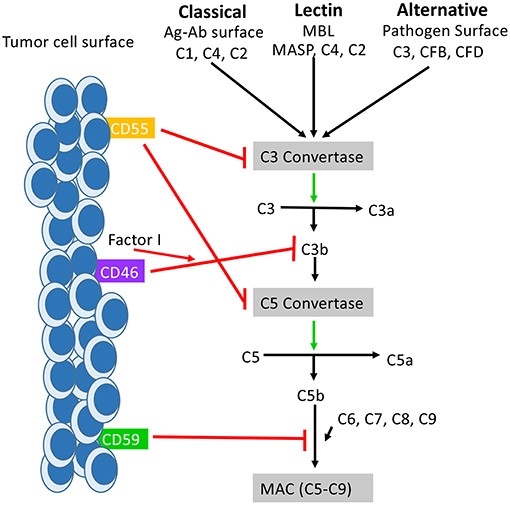 Fig.1 The function of CD55 in the complement system.1,3
Fig.1 The function of CD55 in the complement system.1,3
Beyond its role in complement regulation, studies have revealed that CD55 can function as a co-stimulatory molecule for immune cells and is overexpressed in various tumor types, including colon, breast, and lung cancer. As documented in publications, this overexpression shields cancer cells from immune-mediated destruction, making CD55 a compelling target for oncology therapeutics. Furthermore, CD55's association with diseases like paroxysmal nocturnal hemoglobinuria (PNH) and certain enteroviral infections highlights its broad relevance in medicine, as noted in Frontiers in Immunology articles.
CD55 in Disease Treatment
CD55 has emerged as a significant therapeutic target, especially in oncology, due to its ability to protect cells from immune attack. Its widespread expression on the surface of many cancer cells, including those from colon, breast, lung, and ovarian cancers, makes it a valuable target for novel cancer immunotherapies.
By developing therapeutics that specifically target and inhibit CD55 on tumor cells, we can effectively disable their key defense mechanism. This strategy can be used in two primary ways to enhance cancer treatment:
-
Direct Cell Lysis: Anti-CD55 antibodies can initiate complement-dependent cytotoxicity (CDC), directly killing tumor cells by disrupting their protective shield.
-
Enhanced Efficacy of Other Therapies: Blocking CD55 on tumor cells can significantly improve the effectiveness of other antibody-based treatments that rely on complement activation to destroy cancer cells. This synergistic approach holds great promise for overcoming resistance mechanisms in hard-to-treat malignancies.
Beyond cancer, the complement regulatory role of CD55 makes it an important target for conditions where an overactive complement system contributes to disease pathology. For example, in Paroxysmal Nocturnal Hemoglobinuria (PNH), the absence of GPI-anchored proteins like CD55 on red blood cells leads to their destruction. Recombinant CD55 fusion proteins can provide a therapeutic solution by protecting these cells from complement-mediated hemolysis.
Complement Drugs Targeting CD56
Targeting CD55 offers a promising strategy for modulating the complement system in a highly specific manner. Therapeutic approaches centered on CD55 are designed to either block its function on target cells or to provide a soluble form of the protein to inhibit the complement cascade. This precise control allows for targeted intervention in diseases where CD55 is dysregulated, such as in cancer or certain autoimmune disorders. The main types of drugs in this class are outlined below.
Anti-CD55 Monoclonal Antibodies
These immunoglobulins attach to CD55 surface antigens on malignant cells, including carcinomas. By blocking CD55's function, they make the cells susceptible to complement-dependent cytotoxicity and other immune-mediated destruction.
Recombinant CD55 Fusion Proteins
These engineered proteins, often consisting of a soluble CD55 domain fused to an antibody Fc region, act as a decoy. When administered, they bind to and accelerate the decay of C3 and C5 convertases in the plasma, protecting host cells from an overactive complement response in autoimmune and inflammatory conditions.
Published Data
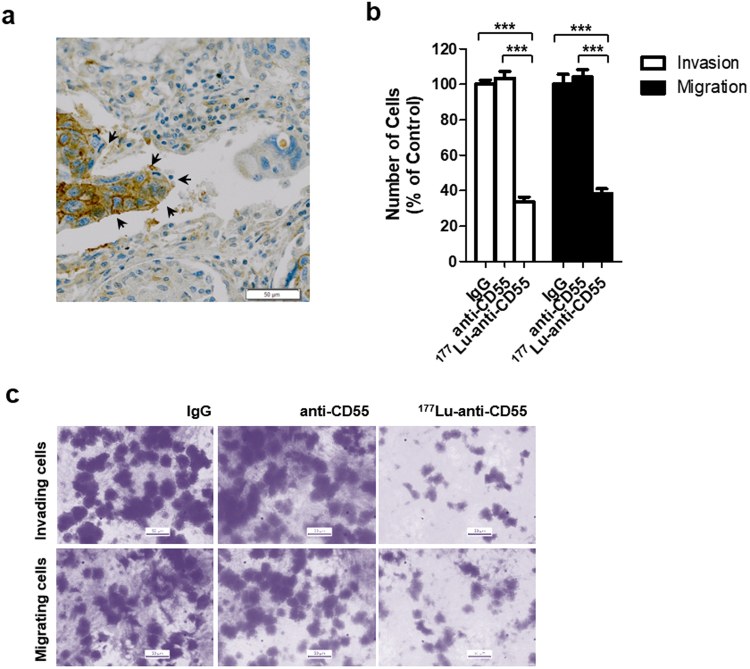 Fig.2 Effects of the 177Lu-anti-CD55 antibody on invasion and migration in lung cancer cells.2,3
Fig.2 Effects of the 177Lu-anti-CD55 antibody on invasion and migration in lung cancer cells.2,3
Non-small cell lung cancer investigations detected heightened CD55 presence in significant human tissue samples. Addressing this occurrence, researchers constructed a lutetium-177-conjugated chimeric monoclonal anti-CD55 antibody. Within a murine model of thoracic cavity metastatic lung cancer, preferential tumor accumulation over healthy tissues was documented. Treatment with the antibody led to a reduction in tumor growth and a doubling of median survival compared to controls. The study also demonstrated that the anti-CD55 antibody enhanced the antitumor activity of cisplatin both in vitro and in vivo, highlighting its potential as a synergistic therapeutic agent. This research underscores our capability to develop highly targeted and effective therapeutics against CD55 in oncology.
What We Can Offer?
Creative Biolabs provides a wide range of products and services to support your research and drug development needs, all designed to streamline your project and maximize your results.
-
Custom Recombinant Protein Production
-
Antibody Discovery & Development
-
Cell-Based Assay Services
-
Preclinical In Vivo Models
-
Complement System Functional Assays (e.g., Hemolytic Assay, ELISA)
-
CD55 (DAF) Protein & Antibody Products
Workflow
01
Target Design & Antigen Preparation: We design the therapeutic molecule and prepare a high-quality recombinant protein or cell line.
02
Antibody/Protein Engineering & Screening: Using advanced platforms, we screen for candidates with desired binding affinity, specificity, and functional activity.
03
In Vitro Functional Assays: We perform assays to test candidate molecules, including complement activation and cell-based cytotoxicity studies.
04
In Vivo Efficacy Evaluation: Promising candidates are moved to animal studies to assess their therapeutic ability and pharmacokinetic profile.
05
Lead Candidate Optimization: Based on in vivo data, we optimize the lead candidate for improved efficacy and manufacturability.
Why Choose Us?
Choosing Creative Biolabs means partnering with a team of experienced professionals dedicated to your project's success. Our Complement System Therapeutic service for CD55 (DAF) offers distinct advantages.
-
Customized Solutions: We provide tailored solutions that go beyond a one-size-fits-all approach, designing a strategy that precisely matches your project goals.
-
Proven Expertise: With extensive experience in complex protein engineering and immunotherapy, our team is adept at navigating the unique challenges of complement-targeted drug development.
-
Cutting-Edge Technology: We utilize state-of-the-art platforms for high-throughput screening, functional assays, and in vivo model development, ensuring the highest quality candidates.
-
Comprehensive Data Packages: Our deliverables are not just molecules; they are robust data packages that provide deep insights into the candidates' properties, accelerating your decision-making process.
-
Relevant Success Stories (Published Data): Our methods have yielded successful outcomes, as evidenced by published data in reputable scientific journals. This track record demonstrates our capability to deliver tangible results.
Obtain the Creative Biolabs Advantage – Seek a Quote Today
FAQs
Q: Why is it important to target CD55 when developing new drugs for diseases like cancer?
A: CD55 acts as a protective shield for both healthy and cancerous cells from complement-mediated destruction. In many tumors, this protein is overexpressed, allowing cancer cells to evade the body's natural immune response. By targeting and inhibiting CD55 on these cells, we can remove this shield, making them vulnerable to complement-dependent cell lysis and enhancing the effectiveness of other cancer treatments.
Q: What is the main difference between targeting CD55 and targeting other complement components like C5?
A: Targeting C5 directly blocks the final stage of the complement cascade, preventing the formation of the Membrane Attack Complex. Targeting CD55, on the other hand, is more focused. It works upstream by regulating the C3 and C5 convertases, and because it is often overexpressed on specific cell types (like tumor cells), it allows for a more targeted therapeutic approach without broadly suppressing the entire complement system.
Q: How can I be sure that the developed therapeutic candidate will be effective in humans?
A: While no preclinical model can guarantee human efficacy, our development process is highly rigorous. We utilize a combination of sophisticated in vitro functional assays and relevant in vivo animal models to thoroughly test and validate each candidate's mechanism of action and therapeutic potential. This data provides a strong foundation for future clinical trials.
Q: Is it possible for a CD55 therapeutic to cause unintended side effects by over-activating the complement system?
A: The design of a CD55 therapeutic is critical. An improperly designed therapeutic could theoretically lead to off-target effects. Our process focuses on developing candidates with high specificity and a well-defined mechanism of action to minimize this risk. We perform extensive in vitro and in vivo safety and toxicology studies to identify and mitigate any potential for unintended complement activation.
Q: How does this service compare to a standard antibody development service?
A: A standard service may provide an antibody that binds to CD55, but our specialized service goes far beyond that. We focus specifically on developing a therapeutic that modulates the complement system, which requires specialized functional assays and a deep understanding of CD55's role in disease. This expertise ensures that the final candidate is not just a binder but a truly functional therapeutic.
Creative Biolabs is a leader in complement system therapeutics, specializing in the development of targeted solutions for CD55 (DAF). Our dedicated team and comprehensive services are designed to help you navigate the complexities of this critical field and bring your innovative project to life.
Featured Services
Feature Products
Click Here to Explore Our Advanced Complement Therapeutics Platform
References
-
Geller, Anne, and Jun Yan. "The Role of Membrane Bound Complement Regulatory Proteins in Tumor Development and Cancer Immunotherapy." Frontiers in Immunology vol. 10 1074. 21 May. 2019, https://doi.org/10.3389/fimmu.2019.01074
-
Dho, So Hee et al. "Development of a radionuclide-labeled monoclonal anti-CD55 antibody with theranostic potential in pleural metastatic lung cancer." Scientific Reports vol. 8,1 8960. 12 Jun. 2018, https://doi.org/10.1038/s41598-018-27355-8
-
Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.
Related Sections:

 Fig.1 The function of CD55 in the complement system.1,3
Fig.1 The function of CD55 in the complement system.1,3
 Fig.2 Effects of the 177Lu-anti-CD55 antibody on invasion and migration in lung cancer cells.2,3
Fig.2 Effects of the 177Lu-anti-CD55 antibody on invasion and migration in lung cancer cells.2,3