Introduction Published Data What We Can Offer? Workflow Why Choose Us? FAQs Featured Services Featured Products
Accelerate Your Research and Development!
Are you currently facing challenges in developing effective immunotherapies for complex diseases like cancer or autoimmune disorders? Creative Biolabs' specialized Complement System Therapeutic: Drug Development Service for CD59 (MIRL) helps you overcome these hurdles by providing a targeted approach to inhibit complement-mediated cellular protection, leveraging advanced recombinant DNA technology and high-throughput screening platforms.
Contact our team to get an inquiry now!
Introduction
The complement system is a vital component of the innate immune system, acting as a cascade of proteins that eliminate pathogens and damaged cells. CD59, also known as membrane inhibitor of reactive lysis (MIRL) or protectin, is a crucial guardian of host cells. It is a glycosylphosphatidylinositol (GPI)-anchored protein widely expressed on the surface of almost all cell types. Its primary function is to inhibit the terminal complement pathway by preventing the formation of the membrane attack complex (MAC), which would otherwise lead to cell lysis.
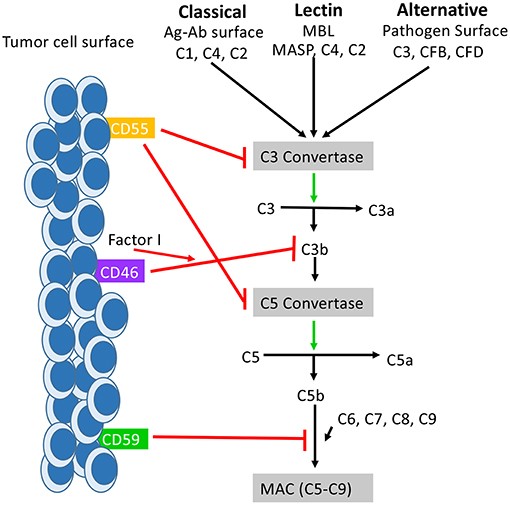 Fig.1 The function of CD59 in the complement system.1,3
Fig.1 The function of CD59 in the complement system.1,3
Scientific literature highlights CD59's role as a major factor in the resistance of cancer cells to complement-dependent cytotoxicity (CDC), a key mechanism of many therapeutic antibodies. By upregulating CD59 expression, tumor cells can evade the immune response. For example, a study showed that a human CD59 inhibitor, rILYd4, could enhance the CDC of anti-CD20 antibodies against drug-resistant B-cell lymphomas. This underscores CD59's critical function in disease pathogenesis and its potential as a compelling therapeutic target. Targeting CD59 can effectively disarm a cancer cell's defense mechanism, making it susceptible to complement-mediated killing.
Complement Drugs Targeting CD59
Complement-targeting drugs are gaining traction for treating a range of conditions, from rare diseases to common ones like autoimmune diseases and certain cancers. These drugs work by inhibiting various steps of the complement cascade. Different types of complement inhibitors target different proteins and pathways. For instance, C5 inhibitors bind to complement protein C5 to prevent its cleavage into C5a and C5b, thereby blocking the formation of the MAC, a therapeutic approach for conditions like PNH and myasthenia gravis. Similarly, C1s inhibitors block the C1s protease in the classical pathway and are used in conditions such as cold agglutinin disease. Factor B inhibitors block the alternative pathway by inhibiting Factor B, a key component of the C3 and C5 convertases, a strategy used for PNH.
Our services are specifically designed to assist in the development of CD59 inhibitors. These drugs work by binding to the CD59 protein on the surface of cells, neutralizing its ability to prevent MAC formation. By inhibiting this protective function, these drugs can enhance the efficacy of existing and novel immunotherapies. This is particularly relevant in oncology, where tumor cells often overexpress CD59 to evade complement-mediated killing.
Published Data
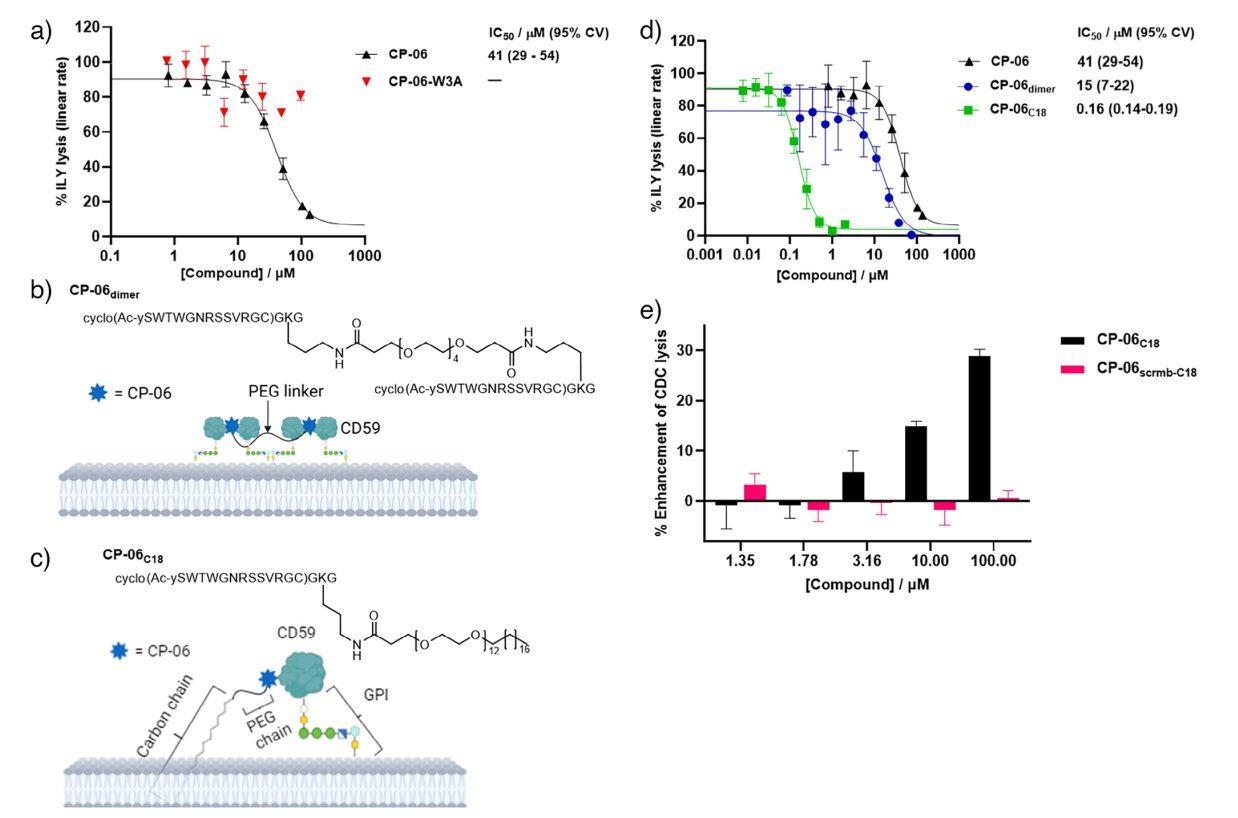 Fig.2 CP-06 peptide and optimized analogues control CD59 activity in cellular assays.2,3
Fig.2 CP-06 peptide and optimized analogues control CD59 activity in cellular assays.2,3
Recently published research highlights the potential of macrocyclic peptides as a new class of CD59 inhibitors. Researchers screened a vast library of cyclic peptides against CD59 to identify potent candidates. Through X-ray crystallography and structure-activity relationship (SAR) studies, they determined that the lead peptide, CP-06, binds to CD59 by mimicking natural protein-protein interactions. The study successfully created dimeric and lipidated versions of the peptide to enhance their cellular activity. The results demonstrated that these probes were effective at inhibiting complement-mediated killing of human cells and blocking the activity of bacterial virulence factors. This work confirms the therapeutic potential of targeting CD59 and supports the feasibility of developing novel small-molecule or peptide-based inhibitors.
What We Can Offer?
Our comprehensive suite of services and products is designed to support every stage of your complement therapeutic drug development project.
-
CD59 (MIRL) Antibody Development: Custom therapeutic antibody development.
-
Complement Functional Assays: Assays to test complement activity, including MAC inhibition and CDC.
-
CD59 Related Proteins: High-quality recombinant CD59 proteins for research and screening.
-
Aptamer Development Services: In vitro services to develop high-affinity aptamers targeting CD59.
-
Formulation Development: Services to optimize stability and delivery of lead antibodies.
Workflow
01
Target Validation & Antigen Preparation: We verify therapeutic potential and prepare high-quality CD59 antigen.
02
Antibody Library Screening: Advanced platforms screen large libraries to identify diverse antibody candidates with high affinity.
03
Functional Assays & Inhibition Testing: Rigorous functional assays evaluate candidates for effective CD59 inhibition.
04
Lead Candidate Characterization: Top candidates are characterized for affinity, specificity, and manufacturability.
05
In Vivo Efficacy Testing (Optional): We can assist with preclinical studies to evaluate in vivo efficacy and safety.
Why Choose Us?
At Creative Biolabs, our deep expertise in the complement system and targeted immunotherapies sets us apart. Our integrated service offerings and a proven track record ensure a streamlined and successful project for our clients.
-
Unparalleled Expertise: Over two decades of expertise in biochemistry and antibody engineering.
-
Innovative Platforms: State-of-the-art platforms for high-throughput screening and functional validation.
-
Comprehensive Service: A full-service solution from target analysis to lead characterization.
-
Published Data: Our methodologies are supported by published data, demonstrating effective and reproducible results.
Obtain the Creative Biolabs Advantage – a href="contact.htm" rel="nofollow">Seek a Quote Today
FAQs
Q: How do CD59 inhibitors specifically enhance cancer immunotherapy?
A: Cancer cells frequently overexpress CD59 to protect themselves from complement-mediated killing, a primary mechanism of action for many therapeutic antibodies. Our CD59 inhibitors work by binding to and neutralizing this protective protein on the cell surface. This action effectively disarms the cancer cell's defense, making it susceptible to the patient's own immune system and therapeutic antibodies. By disrupting the terminal complement cascade at the level of MAC formation, we restore and amplify the efficacy of existing and novel immunotherapies.
Q: What are the key stages in the development of a CD59-targeting antibody, and how does this affect the timeline?
A: The development timeline is influenced by the complexity of each stage. We begin with antigen preparation and advanced antibody library screening to identify a diverse pool of potential candidates. The most critical phase is functional validation, where we perform rigorous in vitro assays to confirm the candidates' ability to effectively inhibit CD59. Finally, we move to lead candidate characterization, which involves detailed analysis of binding kinetics, specificity, and manufacturability. This structured, step-by-step process ensures efficiency, with typical project completion in a few months depending on the scope.
Q: Given that CD59 is widely expressed, how do you ensure your inhibitors do not cause off-target toxicity to healthy cells?
A: This is a core consideration in our drug design. We mitigate this risk through several strategic approaches. First, we develop highly specific antibodies that demonstrate minimal cross-reactivity with other proteins. Second, we utilize advanced functional assays during the preclinical phase to meticulously profile potential off-target effects and ensure a favorable therapeutic index. We can also explore conditional activation strategies where the inhibitor's activity is maximized only within the tumor microenvironment.
Q: What are the most important scientific metrics used to evaluate the potential of a CD59 inhibitor candidate?
A: Beyond simple binding affinity, the most crucial metrics involve functional assays. We use a Complement-Dependent Cytotoxicity (CDC) assay to directly measure the inhibitor's ability to sensitize target cells to complement-mediated lysis. We also use biophysical techniques like Surface Plasmon Resonance (SPR) to precisely measure binding kinetics, including the dissociation constant (KD), and the association (kon) and dissociation (koff) rates. These data provide a comprehensive understanding of the candidate's potency and longevity of effect.
Q: How do antibody-based CD59 inhibitors compare to small-molecule inhibitors in terms of their therapeutic profile?
A: Antibody-based inhibitors offer distinct advantages for targeting a protein like CD59. They possess a high degree of specificity, significantly reducing the likelihood of off-target interactions and associated toxicity. Their large size and stability contribute to a long half-life in the bloodstream, allowing for less frequent dosing. In contrast, small molecules often have a broader target profile and can be cleared more rapidly from the body, which may compromise sustained therapeutic efficacy. Our focus on antibody development leverages these advantages for optimal therapeutic outcomes.
Featured Services
Feature Products
References
-
Geller, Anne, and Jun Yan. "The Role of Membrane Bound Complement Regulatory Proteins in Tumor Development and Cancer Immunotherapy." Frontiers in Immunology vol. 10 1074. 21 May. 2019, https://doi.org/10.3389/fimmu.2019.01074
-
Bickel, Jasmine K et al. "Macrocyclic Peptide Probes for Immunomodulatory Protein CD59: Potent Modulators of Bacterial Toxin Activity and Antibody-Dependent Cytotoxicity." Angewandte Chemie (International ed. in English) vol. 64,27 (2025): e202422673. https://doi.org/10.1002/anie.202422673
-
Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.
Related Sections:

 Fig.1 The function of CD59 in the complement system.1,3
Fig.1 The function of CD59 in the complement system.1,3
 Fig.2 CP-06 peptide and optimized analogues control CD59 activity in cellular assays.2,3
Fig.2 CP-06 peptide and optimized analogues control CD59 activity in cellular assays.2,3