Product List Background AML cell Aptamer Analysis
Background
Acute Myeloid Leukemia (AML)
Acute myeloid leukemia (AML) is a rapidly progressing cancer of the bone marrow and blood marked by the rapid proliferation of abnormal myeloid cells. Symptoms include fatigue, easy bruising, and increased susceptibility to infections. AML develops due to genetic mutations that disrupt average blood cell production, leading to immature cell accumulation. AML disrupts normal blood cell production and can rapidly infiltrate organs like the spleen and liver. Disease development is rapid, often leading to life-threatening complications if untreated. Subtypes of AML are classified based on specific genetic abnormalities and morphological features of the blasts, including myeloid leukemia and acute monocytic leukemia. Current treatments include chemotherapy, targeted therapy, and bone marrow transplantation, tailored based on patient age, overall health, and genetic profile. Despite advancements, challenges remain in managing relapsed or refractory disease and minimizing treatment-related toxicities, highlighting ongoing research efforts to improve outcomes for AML patients.
AML Cell
AML involves a range of cytological abnormalities affecting myeloid progenitor cells in the bone marrow. In normal hematopoiesis, myeloid progenitors undergo a tightly regulated maturation process to form functional blood cells. However, in AML, genetic mutations disrupt this process, leading to abnormal proliferation and differentiation of myeloid precursors that block maturation, boost proliferation, and shield them from apoptosis. These mutations contribute to the heterogeneity observed in AML, influencing disease presentation and response to treatment. Specific mutations, such as in FLT3, NPM1, and CEBPA genes, are associated with distinct AML subtypes and have been targeted in therapeutic research. Numerous AML cell lines with specific mutations have been established, including HL-60 (promyelocytic, often used for differentiation studies), THP-1 (monocytic), and MOLM-13 (FLT3-ITD mutation), facilitating targeted research on AML pathogenesis and therapy development.
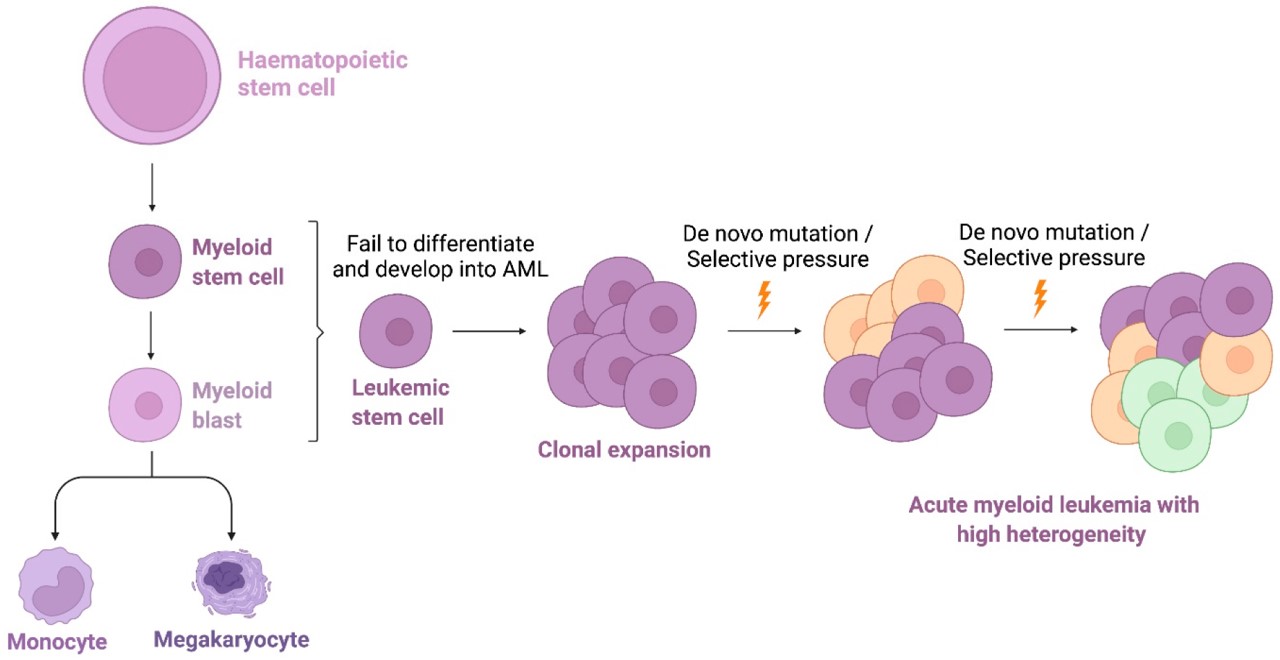 Fig.1 The cells where AML develops.1,4
Fig.1 The cells where AML develops.1,4
Signaling Proteins and Pathways of AML Cells
AML cells commonly involve dysregulation of several key signaling pathways and proteins crucial for cell survival and proliferation. The Ras/Raf/MEK/ERK pathway is frequently activated, promoting AML cell growth and survival through downstream effectors that regulate gene expression. The PI3K-Akt-mTOR pathway is activated in AML cells, which may contribute to metabolic reprogramming and AML cell survival. JAK/STAT signaling, activated by cytokine receptors, controls cell proliferation and differentiation, impacting leukemogenesis in AML. Mutations in FLT3, NPM1, and RAS genes often drive AML pathogenesis by constitutively activating these pathways.
Targeted Therapy in AML
Targeted therapy offers a promising approach to cancer treatment by delivering chemotherapy directly to cancer cells, minimizing side effects on normal tissues, and enhancing treatment effectiveness. Targeted therapy in AML focuses on inhibiting specific signaling proteins and pathways critical for leukemic cell survival and proliferation. Small molecule inhibitors, like FLT3 inhibitors, target mutated kinases to disrupt downstream signaling cascades essential for AML progression. Monoclonal antibodies bind to surface antigens on AML cells, delivering cytotoxic agents directly. Aptamers, synthetic oligonucleotides, can also target AML-specific markers, offering potential therapeutic precision. Ongoing research aims to enhance the specificity and efficacy of these therapies while minimizing off-target effects and advancing personalized treatment options for AML patients.
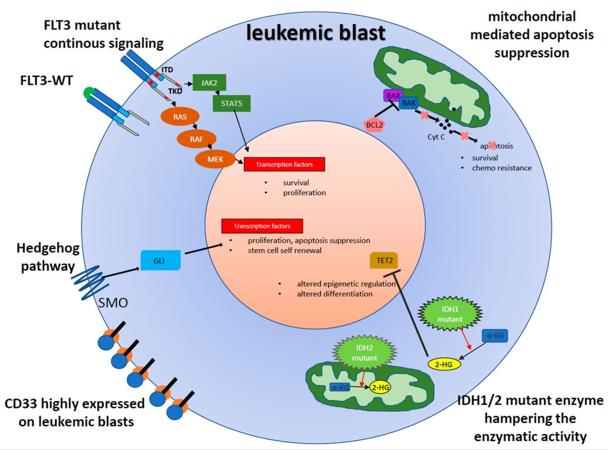 Fig.2 The aberrant and potentially druggable signaling in leukemic blasts.2,4
Fig.2 The aberrant and potentially druggable signaling in leukemic blasts.2,4
Creative Biolabs offers several aptamers targeting AML cells which provide high affinity and low immunogenicity, enhance research accuracy, and assist in your projects.
AML cell Aptamer Analysis
Anti-AML cell aptamers are short, single-stranded oligonucleotides or peptides that specifically bind to AML cells. Their potential in diagnosing, treating, and monitoring AML has driven significant research progress in recent years, representing a significant area of research in cancer therapeutics.
Anti-AML cell aptamers primarily target surface markers or specific biomarkers expressed on AML cells, such as CD33, CD117, CD123, and OFA/iLRP, which are commonly overexpressed in AML. Aptamers can selectively bind to these targets, enabling precise detection and analysis of AML cells. Their ability to bind with high specificity also makes them powerful tools for therapeutic applications. They can inhibit AML cell growth by blocking essential surface receptors involved in cell proliferation and survival.
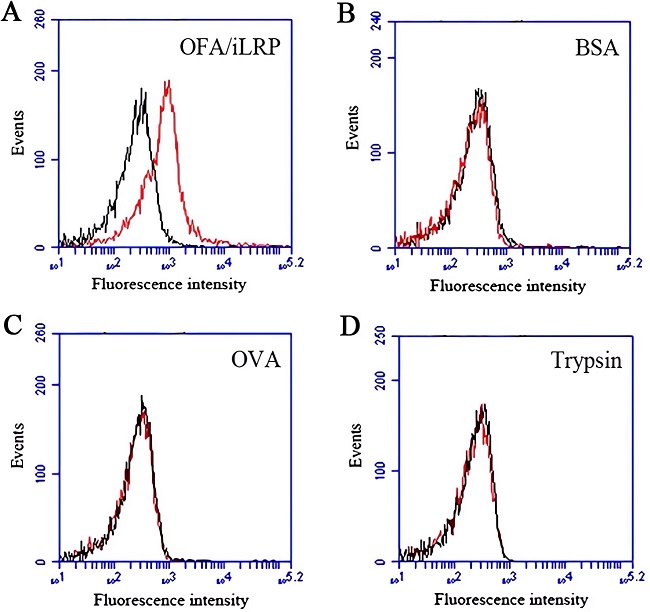 Fig.3 Binding properties of DNA aptamer against OFA/iLRP.3,4
Fig.3 Binding properties of DNA aptamer against OFA/iLRP.3,4
Furthermore, anti-AML aptamers can be conjugated with drugs or toxins, forming aptamer-drug conjugates (ADCs) to deliver cytotoxic agents directly to leukemia cells, minimizing off-target effects. This targeted delivery system improves treatment efficacy while minimizing the side effects typically linked to conventional chemotherapy. Anti-AML aptamers may also be utilized for targeted immunotherapy, increasing the precision of cancer treatments. Studies have shown promise in using aptamers for imaging and as part of immunotherapy strategies to improve patient outcomes.
Creative Biolabs provides comprehensive services related to anti-AML cell aptamers, including aptamer discovery, optimization, and binding assays. With our expertise and advanced platform, our customers can gain access to cutting-edge aptamer technologies, empowering them to accelerate their AML research innovations.
References
-
Xiang, Wei, et al. "Application of high throughput technologies in the development of acute myeloid leukemia therapy: Challenges and progress." International Journal of Molecular Sciences 23.5 (2022): 2863.
-
Bohl, Stephan R., Lars Bullinger, and Frank G. Rücker. "New targeted agents in acute myeloid leukemia: new hope on the rise." International Journal of Molecular Sciences 20.8 (2019): 1983.
-
An, Yacong, et al. "Selection of a novel DNA aptamer against OFA/iLRP for targeted delivery of doxorubicin to AML cells." Scientific Reports 9.1 (2019): 7343.
-
Distributed under Open Access license CC BY 4.0, without modification.


 Datasheet
Datasheet Fig.1 The cells where AML develops.1,4
Fig.1 The cells where AML develops.1,4
 Fig.2 The aberrant and potentially druggable signaling in leukemic blasts.2,4
Fig.2 The aberrant and potentially druggable signaling in leukemic blasts.2,4
 Fig.3 Binding properties of DNA aptamer against OFA/iLRP.3,4
Fig.3 Binding properties of DNA aptamer against OFA/iLRP.3,4
