Adenoviral Vector-based Vaccine Development
Adenoviruses have evolved from tools for gene replacement therapy to vaccine delivery vehicles. They are attractive vaccine vectors as they induce both innate and adaptive immune responses in mammalian hosts. Adenovirus vectors have been tested as subunit vaccine systems for numerous infectious diseases ranging from malaria to HIV-1. Creative Biolabs is a world-class biotechnology company and has developed a comprehensive adenoviral vector construction platform for gene therapy. We offer a variety of adenoviral vector construction services to meet the needs of each customer.
Adenoviral Vector Vaccine
Adenoviruses Introduction
Adenoviruses were initially vectored as vehicles for gene therapy. Attempts to replace missing or faulty genes by adenovirus gene transfer have largely failed due to adenovirus-induced innate and adaptive immune responses. This reduced their appeal as gene replacement vehicles while it invited their use as vaccine carriers. Thus far, most efforts have focused on vectors derived from adenovirus of the human serotype 5 (AdHu5) and a large proportion of adenovirus vectors tested in the clinic are based on this serotype.
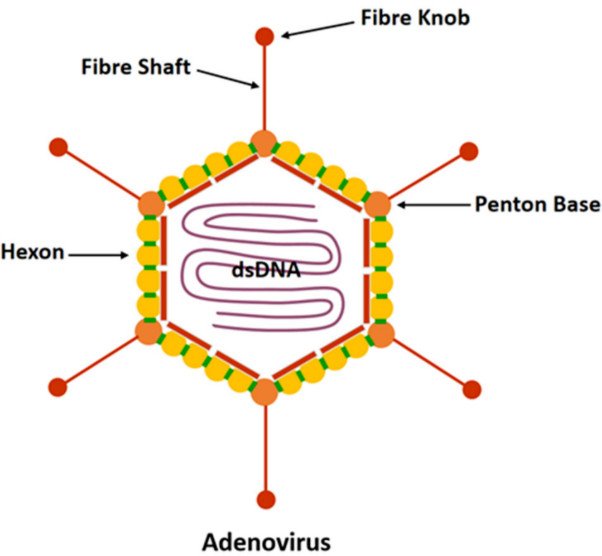 Figure 1 Human adenovirus structure.1
Figure 1 Human adenovirus structure.1
Vaccine Introduction
Vaccines based on adenovirus (Ad) vectors are the cornerstone of modern vaccine science. This technology platform utilizes genetically modified adenovirus as a safe carrier to deliver specific gene fragments from pathogens to human cells. These vaccines are highly praised for their strong immunogenicity, excellent safety, and efficient production capacity, and have become the main tools for developing preventive vaccines and innovative immunotherapies.
Adaptive Immunity Induced by Adenoviral Vectors
Vaccines based on adenovirus vectors are known for their ability to induce strong humoral (antibody mediated) and cellular (T cell-mediated) immune responses.
- Humoral immunity: Foreign antigens expressed are secreted or presented on the surface of cells. These antigens are recognized by B cells, which differentiate into plasma cells with the support of helper T cells and produce antigen-specific antibodies. These antibodies can neutralize pathogens and prevent them from infecting other cells.
- Cellular immunity: Antigens produced within cells are processed and presented on the cell surface through the major histocompatibility complex (MHC) class I pathway, which is crucial for activating cytotoxic T lymphocytes (CTLs). These cytotoxic T cells are crucial for clearing infected cells and providing a vital protective layer against intracellular pathogens.
Comparison of Adenoviral Vector Generations
| Generation | Genetic Modifications | Insert Capacity | Immunogenicity | Applications |
|---|---|---|---|---|
| First-generation | E1 deletion, sometimes E3 deletion | 4-5 kb | High | Proof-of-concept studies, cancer vaccines |
| Second-generation | Additional E2 or E4 deletions | 8-10 kb | Moderate | Infectious disease vaccines, longer-term expression needed |
| Third-generation (Helper-dependent) | All viral coding sequences deleted | Up to 37 kb | Low | Large gene inserts, long-term expression required |
Antigen Presentation Mechanisms of Adenoviral Vector-Based Vaccines
The potent immunogenicity of adenoviral vectors is directly linked to their sophisticated antigen presentation mechanisms. The strong immunogenicity of adenovirus vectors is directly related to their complex antigen presentation mechanism.
MHC Class I Antigen Presentation (Cross Presentation)
After the vector is detached from the intracellular body, the expressed antigen enters the cytoplasm of the host cell. Although some antigens are directly processed and presented to MHC class I molecules, an important aspect of adenovirus vector immunity is the cross-presentation process. Here, infected antigen-presenting cells (APCs) take up antigens from other infected or dying cells and present them to their own MHC class I molecules to activate immature CD8+T cells. This mechanism is highly efficient and can generate strong and persistent CTL responses.
MHC Class II Antigen Presentation
Virus particles and expressed antigens are also taken up by APCs and transported to the intracellular lysosome pathway. Here, the antigen is processed and loaded onto MHC class II molecules, and then transported to the cell surface. This presentation of MHC class II is crucial for activating helper T cells (CD4+T cells), which provide necessary support for the activation and maturation of B cells and the production of strong antibody responses.
Core Services at Creative Biolabs
As more effective, novel and safe vaccines are sought for existing human diseases, adenovirus vectors are becoming an important immuno-prophylactic tool in modern medicine. Adenoviruses have a broad tropism infecting a variety of dividing and non-dividing cells. They can be grown to high titers in tissue culture. The advantages of adenovirus vectored vaccines include efficient antigen presentation and induction of both humoral and cell-mediated immunity. Adenovirus vectors are being extensively explored for their applications in gene therapy. Based on high-end scientists and professional platforms, we provide the AdHu5 and other serotypes of adenovirus vector construction services for vaccine development.
- Vector design and construction: Customize vector design using multiple serotypes and promoters to optimize expression.
- Purification and characterization of vector : Production and purification of research grade and clinical grade carriers, and comprehensive quality control.
- Immunogenicity assessment: In vitro and in vivo studies to evaluate the humoral and cellular immune responses induced by candidate vaccines.
Our Service Workflow
Our streamlined workflow ensures a smooth and efficient project from conception to completion.
- Consulting and Design: Our professional scientists will work hand in hand with you to understand your project goals and design the best adenovirus vector.
- Vector Construction: Clone the target gene into our proprietary adenovirus backbone.
- Vector Production and Purification: We use large-scale production and advanced purification technology to produce high-purity and efficient carriers.
- Quality Control and Delivery: The final product will undergo strict quality control checks before delivery to ensure its sterility, characteristics, and purity.
Adenoviral Vector Technology Platforms
Creative Biolabs has established a multi serotype adenovirus vector technology platform, covering C-type adenovirus (Ad2, Ad5), B-type adenovirus (Ad35, Ad11), and non-human adenovirus (such as chimpanzee adenovirus ChAdOx1). This platform integrates high-throughput vector design, vector production based on rod-shaped viruses, and quality control (QC) systems.
Table 2. Key Characteristics of Adenoviral Vector Vaccine Platform
| Characteristic | Advantage | Application Benefit |
|---|---|---|
| Broad cellular tropism | Efficient transduction of antigen-presenting cells | Potent immune response initiation |
| High transduction efficiency | Robust antigen expression at low doses | Reduced vaccine dosage requirements |
| Large transgene capacity | Accommodation of multiple antigens or complex genes | Multi-valent vaccine development |
| Episomal persistence | No risk of insertional mutagenesis | Enhanced safety profile |
| Innate immunogenicity | Built-in adjuvant effect through viral components | Reduced need for exogenous adjuvants |
| Production scalability | High-titer manufacturing capability | Rapid response to pandemic threats |
Our Advantages
Choose Creative Biolabs to provide multiple unique advantages for your adenovirus vector vaccine project:
- Deep research capabilities: Our team of doctoral level scientists has extensive experience in virology, immunology, and vaccine development.
- Comprehensive platform: We provide a one-stop solution from gene synthesis to immunogenicity testing.
- Fast and efficient service: Our optimized workflow and advanced technology ensure that projects are completed quickly.
- Customization and flexibility: We customize services based on your specific research needs and project scale.
Frequently Asked Questions
Q: What are the main safety features of your adenovirus vector?
A: Our vector is replication defective, achieved by deleting the E1 gene. This can prevent the vector from replicating in the host's body and causing disease, thereby ensuring a high safety factor.
Q: Can you use different adenovirus serotypes?
A: Yes, we offer multiple serotypes, including HAdV-5, HAdV-26, and chimpanzee adenovirus vectors, to address pre-existing immune issues and optimize immunogenicity based on your specific application.
Q: How does adenovirus vector induce T cell immunity?
A: Adenovirus vectors effectively deliver antigen genes to antigen-presenting cells (APCs). The expressed antigen is then processed and presented to MHC class I molecules, thereby activating cytotoxic T lymphocytes (CTLs), which are crucial for clearing infected cells.
Q: What is the maximum transgenic size that adenovirus vectors can carry?
A: The cloning capacity depends on the type of vector:
- E1/E3 deletion vector: approximately 8 kb (can be expanded to approximately 10 kb by deleting other non-essential genes such as E4).
- Assisted Dependent Adenovirus Vector (HDAdV): Approximately 36 kb, as all viral coding sequences have been deleted.
- For transgenes larger than 8 kb, we recommend using HDAdV. Our team has successfully constructed HDAdV carrying a 30 kb transgenic gene (such as a multi antigen HIV vaccine).
Q: Can these vectors be used for diseases that require long-term antigen expression, such as genetic diseases?
A: Although adenovirus (AdV) does not integrate and its expression in dividing cells is usually short-lived (weeks to months), they can mediate long-term expression in non-dividing cells. In order to truly correct genetic diseases in the long term, lentiviral or adeno-associated virus (AAV) vectors may be more suitable. We will provide guidance on selecting the best platform based on your specific application.
Connect with Us Anytime!
Creative Biolabs has been dedicated to the development of gene therapy for many years. We specialize in serotype switching and capsid engineering. By utilizing rare human serotypes or chimpanzee-derived adenoviruses, we circumvent the issue of pre-existing immunity prevalent in populations for common serotypes like Ad5. Our proprietary shuttle vector systems and direct cloning techniques accelerate vector construction from months to weeks. If you are interested in our services, please contact us for more details.
Reference
- Yang K, Feng S, Luo Z. Oncolytic adenovirus, a new treatment strategy for prostate cancer. Biomedicines, 2022, 10(12): 3262. https://doi.org/10.3390/biomedicines10123262 (Distributed under Open Access license CC BY 4.0, without modification.)
