Hemophilia A/B
Hemophilia A/B is a group of hemorrhagic diseases with hereditary coagulopathy. Its common feature is the active thromboplastin-producing disorder with prolonged clotting time, and a tendency to post-traumatic hemorrhage in life. Patients often show a lack of the F8 (hemophilia A) or F9 (hemophilia B) genes, based on which, scientists focus on gene therapy to prolong patient's survival time. The most typical case in clinical research is to design adeno-associated virus (AAV) vector, a single-stranded small DNA virus, for the delivery of factor VIII or IX to hepatocytes. Creative Biolabs provides a full range of technical services, including vector preparation, gene loading, gene expression and efficacy validation to satisfy your needs.
Research Progress of Hemophilia
Hemophilia is a clinically recurrent bleeding disease with a high mortality rate. Since the first distinguish of the genes F8 (hemophilia A) or F9 (hemophilia B) in the 1950s, gene therapy of Hemophilia has made great progress. The first clinical treatment for hemophilia A was by intravenous injection of AAV vectors to inject in the omentum during a laparoscopic procedure. The entire process did not achieve long-term results, although it was non-toxic for patients. However, for hemophilia B, doctors transplanted the AAV vectors into the liver of the patient by interventional therapy, with clinical efficacy lasted for several weeks, which lays a solid foundation for subsequent liver transmission.
- Hemophilia A
Gene therapy for hemophilia A is still in the early stages, which needs further research and development by researchers. It has been reported that optimizing codons in the coding region can increase gene F8 expression by up to 40 times, however, this non-wild-type sequence tends to stimulate the antigenicity of the body. Fortunately, the design of AAV vectors for liver-mediated gene therapy has made some progress in hemophilia A dogs.
- Hemophilia B
The size of hemophilia B is about 4.4 kb even for the B-domain-deleted construct, however, due to the structure of AAV itself, AAV vectors cannot insert more than 5 kb genes, which has become a drawback for AAV vectors in the treatment of hemophilia B. Investigators at St Jude Children's Research Hospital and University College London packaged a vector genome into an AAV8 capsid, promoting a significant increase in factor F9 levels, which efficiently relieves the patient's bleeding condition and reduces the pain of the patient. Moreover, scientists attribute the massive replication of the factor F9 to the activation of T cells by the AAV vector, which causes the body to produce strong immune responses. These results fully demonstrate that AAV vectors can be used for gene therapy of hemophilia B and have achieved objective results.
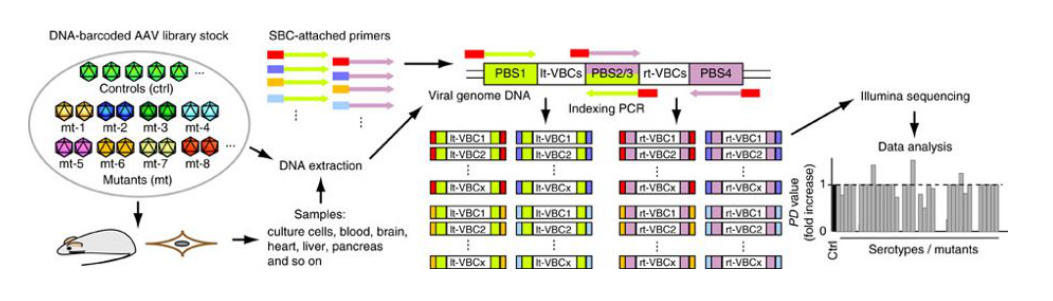 Figure 1. Procedure for the AAV Barcode-Seq analysis. (Adachi, 2014)
Figure 1. Procedure for the AAV Barcode-Seq analysis. (Adachi, 2014)
With the rapid development of gene therapy, significant results have been achieved with the use of AAV vectors for hemophilia therapy. Creative Biolabs owns the most scientific AAV carrier design and preparation methods. We will offer the best services to support the theoretical basis and scientific guidance in the clinical treatment of hemophilia. For more information, please contact us in time and we will be happy to serve you.
Reference
- Adachi, K.; et al. (2014). Drawing a high-resolution functional map of adeno-associated virus capsid by massively parallel sequencing. Nature Communications. 5: 3075-3099. Distributed under Open Access license CC BY 4.0, without modification.
