AAV Vector Design Service for Pompe Disease
Pompe disease is an autosomal recessive neuromuscular disorder caused by acid alpha-glucosidase (GAA) lysosomal enzyme deficiency. Infantile-onset Pompe disease is the most severe form of the disorder and patients start to manifest the symptoms of cardiomyopathy, respiratory failure, and skeletal muscle weakness within weeks after birth. Late-onset Pompe disease patients exhibit a slower disease progression while still developing skeletal muscle weakness. Despite therapeutic advancements for Pompe disease management current enzyme replacement therapies continue to face challenges including immune system reactions against the enzyme restricted enzyme mobility across the blood-brain barrier and the need for ongoing treatment throughout patients' lifetimes. AAV vectors mediated gene therapy may be able to provide better bioavailability of the transgene product to increase efficacy. For in vivo AAV gene therapy, several considerations, such as dosing amount, biodistribution, and vector and transgene product immunogenicity, should be carefully evaluated. The development of the vector design and the transgene product may address some of these limitations by reducing the risk of vector-induced immunogenicity and increasing efficacy. Alternatively, gene therapy could provide the necessary systemic delivery of the vector and transgene product, with improvements in HSPC conditioning and transduction efficiency to maximize engraftment of modified cells in the periphery and central nervous system for improved efficacy. A key consideration for disease background is in treatment modalities where there could be a large difference between the benefit and risk ratio associated with the therapeutic strategy necessitating that every critical step in the preclinical development phase be meticulously scrutinized. Gene therapies have the potential to be a single-dose therapy that would allow for lifelong therapeutic GAA expression with the potential to prevent, stop, or reverse Pompe disease.
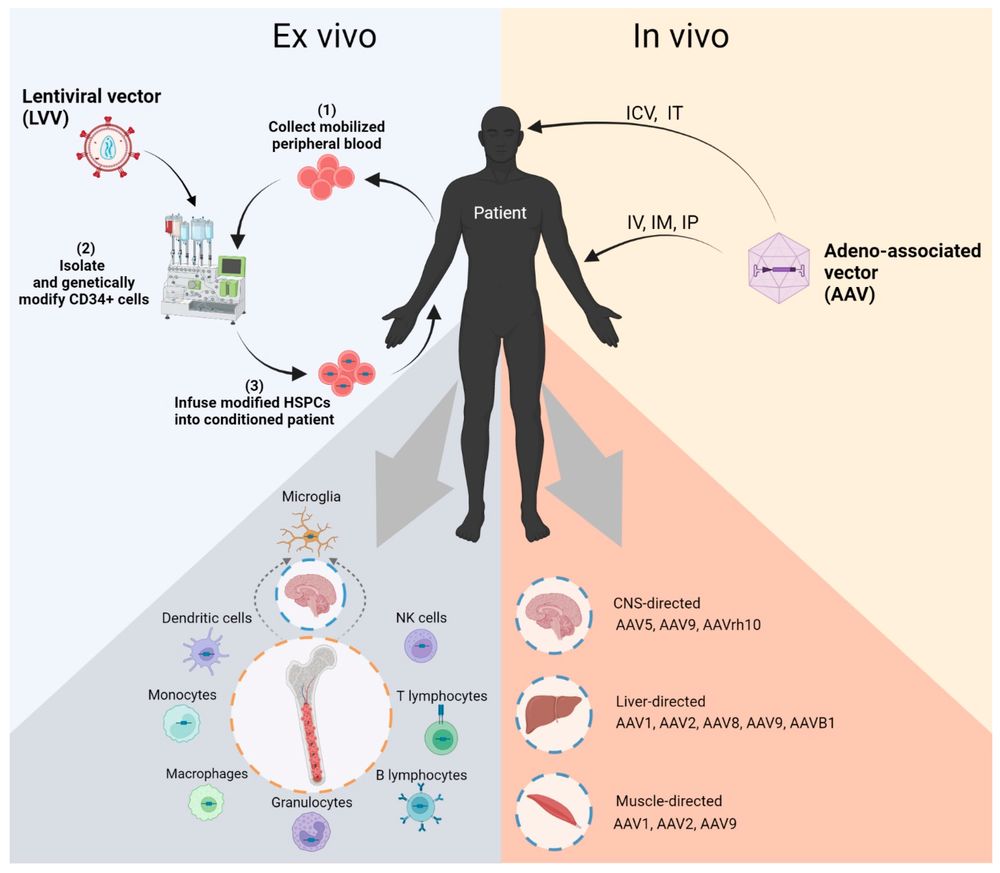 Fig.1 Overview schema of Pompe disease gene therapy modalities1,2
Fig.1 Overview schema of Pompe disease gene therapy modalities1,2
Our guiding principle is that enduring therapeutic action should not result in indelible marks on the genome. Creative Biolabs's AAV vector design service for Pompe disease integrates a profound understanding of gene therapy dynamics, structure-guided capsid engineering, and targeted transgene expression into a cohesive process that streamlines the journey from your genetic inquiry to a clinically robust, AAV-delivered enzyme replacement therapy. Leveraging serum-free, suspension-based packaging systems fine-tuned for optimal vector yield, we harness an array of advanced molecular tools, including combinatorial promoter selection, capsid surface modification, and pH-sensitive fusion mechanisms to engineer vectors of exceptional potency, with minimal risk of off-target effects, and activation of enzyme expression exclusively within the diseased muscle tissue. These precisely designed vectors, devoid of endotoxins and aggregates, brightly illuminate the glycogen-laden muscle cells in patient-specific models, facilitating a swift advancement from laboratory insights to peer-reviewed findings, all without leaving a lasting genomic imprint or causing delays in your research trajectory.
Your Strategic Advantage in Gene Therapy
Vector development doesn't have to be a stumbling block. We bring expertise, advanced technology and a partnership you can count on to help you overcome challenges and achieve objectives faster.
Allied Expertise in Vector Engineering
Our team of expert scientists serves as a valuable asset to your team, offering forward-thinking advice on vector configuration, enhancement, and regulatory compliance strategies.
Growth-Oriented Platform
Our system is designed to expand with your needs, ensuring reliable vector quality and effectiveness as you transition from initial research to extensive pre-clinical studies.
Commitment to Excellence and Security
Adhering to a principle of prioritizing safety and implementing stringent quality control measures, we provide vectors that are fully compliant with the rigorous standards necessary for pre-clinical and clinical advancements.
Efficiency-Focused Project Coordination
We recognize the importance of pace in your endeavors. Our dedicated project managers guarantee clear communication and punctual delivery, ensuring that your project remains on course.
Workflow: A Clear Path from Concept to Delivery
Our transparent, phase-gated process ensures quality and consistency at every step, providing you with clear milestones and predictable outcomes.
| Service Module | Deliverables | How Obtained |
|---|---|---|
| Design of AAV Vectors | Detailed GenBank files, Restriction maps, Regulatory logic reports | Use bioinformatics software to create annotated nucleotide sequences. Mapping restriction enzyme sites across the vector sequence. Reports is prepared by analyzing regulatory elements and their impact on vector function. |
| Vector Construction and Optimization | High-purity plasmid DNA, QC reports, Plasmid master file template | Use a combination of alkaline lysis and column chromatography techniques. Through gel electrophoresis and spectrophotometry to assess DNA purity and concentration. Plasmid master file template from detailed records of all construction steps and quality checks. |
| Vector Purification | Purified AAV vectors, Real-time empty/full capsid ratio analysis, validation reports | Vectors are obtained through ultracentrifugation and iodixanol gradient purification. Conducted using dynamic light scattering or capsid protein assays. Prepared by assessing vector integrity and functionality through various assays. |
| Vector Titration | Accurate titer reports, Raw data CDs, Regulatory readiness | Reports are determined by quantitative PCR or plaque assays to measure vector concentration. Data from all experimental data collected during titration. Analysis is ensured by documenting all procedures and data in compliance with regulatory standards |
| Toxicity/Safety Determination | Safety reports, NOAEL data, Integration mapping reports, Immunogenicity data | Reports on the results of in vitro and in vivo toxicity studies. Data is determined from dose-response studies to find the highest dose without adverse effects. The map is generated by analyzing vector DNA integration sites in host genomes. Results are obtained from immunological assays measuring host immune responses to the vector |
Comprehensive Service Offerings
At Creative Biolabs, we understand the critical need for precise and effective gene delivery in treating Pompe disease. Our AAV vector design service leverages the latest advancements in AAV technology to create vectors optimized for muscle and liver targeting, the primary tissues affected by Pompe disease. Our design process begins with a thorough analysis of the target tissue and disease state, utilizing our proprietary database of AAV serotypes and regulatory elements. This database, updated quarterly with over 4,000 literature entries, provides a comprehensive understanding of AAV tropism and expression dynamics.
Vector Design Service
We recommend AAV9 and AAV8 for muscle and liver targeting, respectively, due to their high transduction efficiency in these tissues. AAV9 has demonstrated exceptional ability to transduce muscle cells, while AAV8 is highly effective in hepatocytes. We utilize tissue-specific promoters to drive high-level expression of the GAA gene specifically in muscle and liver cells. This approach minimizes off-target effects and maximizes therapeutic efficacy. Our vectors also include miRNA-based logic gates and synthetic intracellular amplifiers to precisely regulate gene expression, achieving optimal therapeutic levels of GAA while reducing potential immunogenicity. We provide detailed GenBank files, restriction maps, and regulatory logic reports, ready for pre-submission meetings with regulatory authorities.
Vector Construction
Once the design is confirmed, our GMP-like vector construction platform ensures rapid and high-quality production. Our patented AAV expression backbone library supports expression cassettes up to 4.7 kb, with options for reporter genes (eGFP, luciferase) and selection markers (Puro, Neo, BSD). All cloning procedures are carried out in ISO Class 5 cleanrooms, using culture systems free of antibiotics and animal components to avoid contamination. Plasmid DNA purification from the high-purity plasmid is carried out using Plasmid Mega Kits or TFF membrane filtration for a high level of endotoxin control (<0.1 EU/µg), with a A260/280 ratio of 1.8-2.0. Sequencing is performed in a 100% accurate fashion, using bidirectional Sanger sequencing and NGS validation.
Vector Purification
For Pompe disease applications, high-purity AAV preparations are essential for both safety and efficacy. Our two-tiered purification approach guarantees ≥90% intact capsids for research-grade material using CsCl or iodixanol gradient ultracentrifugation. Clinical-grade batches are scaled using high-resolution affinity chromatography and ion-exchange polishing, meeting stringent release specifications for endotoxin (<1 EU/mL), host DNA (<10 ng/dose), and rcAAV (<1/105). Real-time empty/full capsid ratio analysis and ICH Q2(R1) compliant method validation reports are available upon request.
Vector Titration
Accurate genomic titer quantification is crucial for dose calculation and clinical submissions. Our ddPCR platform offers single-copy detection limits and a linear range of 102--108 vg/reaction. We provide three orthogonal methods of analysis (qPCR, ELISA capsid protein quantification, and TCID50 infectivity assay) to cross-validate your data. GMP compliant validation protocols and raw data CDs ensure regulatory readiness.
Virus Production
Our scalable production platforms cater to both research and clinical needs. From research-scale batches to clinical-scale productions, we offer linear scalability in bioreactors. Our Sf9/baculovirus system delivers ultra-high yields with no risk of replication-competent viruses. The HEK293 triple-plasmid transfection system is also available for high production single-use bioreactors for transgenes in need of post-translational modifications. Both platforms are scalable and use serum-free, animal-component free media to allow a closed production system without the risk of cross-contamination.
Toxicity/Safety Determination
Safety is paramount for clinical translation. Our GLP-compliant integrated packages include rapid repeat-dose toxicity studies, biodistribution, and immunogenicity testing in AAALAC-accredited facilities. In rats (Sprague Dawley), we establish NOAEL with no histopathological signs of inflammation, necrosis, or hyperplasia. AAV integration is mapped by whole-genome NGS, with integration hotspots observed in <0.01% of the population. Serum IL-6/IFN-β levels, and IFN-γ+ T cells, all within accepted clinical ranges.
Our Client Testimonials
We are committed to fostering enduring collaborations and proud of our track record for outstanding service delivery. Discover the testimonials from prominent research entities and biopharmaceutical firms regarding our offerings.
"In tackling the complexities of Pompe disease, Creative Biolabs's tailored AAV vectors have been instrumental. Their design expertise has given us a vector that targets muscle tissue with remarkable specificity, significantly enhancing the therapeutic enzyme's efficacy without the fear of genome integration. The seamless collaboration has been a boon to our research efforts."
Dr. Victoria Adams
Lead Researcher
"Addressing the unique challenges of Pompe disease required a vector with high muscle tropism and long-term expression. Creative Biolabs delivered a meticulously designed AAV vector that has proven to be both effective and safe in our preclinical models. Their service has been invaluable in advancing our therapeutic approach."
Dr. Lisa Martinez
Principal Investigator
"We have partnered with Creative Biolabs for their extensive experience in AAV vector design. They have designed vectors with good tissue tropism and long term expression in our Pompe disease models. They are also very professional, and have a great focus on quality."
Prof. Sarah Nguyen
Senior Scientist
"The AAV vectors designed by Creative Biolabs for Pompe disease have provided us with a reliable tool to study enzyme replacement therapy. Their focus on muscle-specific expression and avoidance of genome integration has simplified our research process and given us confidence in the safety of our approach."
Prof. Laura Patel
Head of Gene Therapy Research
Frequently Asked Questions
Have questions? Find quick answers here. For more specific inquiries, please don't hesitate to contact us.
Q: How do you ensure the safety of the AAV vectors for use in Pompe Disease treatment?
A: Safety of the AAV vectors are confirmed by several methods. These methods include safety assessments which look at various factors such as immune responses, off-target effects, and replication-competent AAV (rcAAV). Vector production is also performed under GMP-like conditions to ensure the vectors are of therapeutic quality and do not have any detrimental effects due to vector administration.
Q: What types of muscles are targeted by the AAV vectors in Pompe Disease treatment?
A: AAV vectors target a wide variety of muscle tissues, including both skeletal and cardiac, both of which are primarily affected in Pompe Disease. Targeting can be modified according to disease manifestation and treatment objective.
Q: How do you address the issue of pre-existing immunity to AAV vectors in Pompe Disease patients?
A: The patient's prior immune experience is taken into account and we may suggest the use of other AAV serotypes with a lower seroprevalence of neutralizing antibodies or other immune evasive strategies.
Q: What features are incorporated into the AAV vectors to enhance their therapeutic potential?
A: Several elements are included in our AAV vectors to optimize therapeutic efficacy. These include self-complementary (scAAV) genomes for faster, higher level expression; the WPRE to improve mRNA stability and muscle-specific promoters to restrict the expression. We may also utilize approaches to circumvent pre-existing immunity, including use of alternative serotypes or immune-evasion elements.
Q: Are there any limitations to the AAV Vector Design Service for Pompe Disease?
A: Although our service is highly flexible, there can be limitations to this due to the specific needs of the therapeutic gene or patient population. We discuss these limitations with our clients and explore ways to overcome them, so that the final product can be used in Pompe disease patients as needed for therapy.
Q: How do I initiate a Pompe-specific vector project?
A: Submit to us your desired expression format (intracellular or secreted), target muscle groups and any capsid or promoter limitations. We will respond with a design summary with justification, recommended read-outs for analysis and a fixed price quote. Once accepted, we immediately begin gene synthesis and cloning, keeping you updated every step of the way via a secure data portal.
Q: Can the vector be tailored for cardiac versus skeletal muscle, or for smooth muscle in the vascular wall?
A: Absolutely. We have a panel of promoters (desmin, MHCK7, cTnT variants, SM22α) of variable strength and cell-type stringency. By exchanging these cassettes into the same capsid backbone, we can drive expression with a bias toward diaphragm, soleus, left ventricle or vascular smooth muscle. If your application requires a pan-muscle approach, we can also deliver a chimeric promoter that is active in striated and cardiac tissue. We offer an annotated rationale for the expected expression range of each cassette option so you can select the one that aligns best with your therapeutic hypothesis.
References
- Unnisa, Zeenath, et al. "Gene therapy developments for Pompe disease." Biomedicines 10.2 (2022): 302. https://doi.org/10.3390/biomedicines10020302
- Distributed under Open Access license CC BY 4.0, without modification.
