AAV Vector Design Service for X-linked Duchenne Muscular Dystrophy (DMD)
Introduction
Duchenne Muscular Dystrophy programs face challenges in AAV vector design, immune response and therapeutic efficacy. Creative Biolabs' custom rAAV design service addresses these via advanced engineering and payload optimization, creating safe, effective next-gen therapies. We deliver precision solutions tailored to needs, guiding from genetic data to validated candidates with optimized vectors, enhanced micro-dystrophin and preclinical packages for regulatory support.
Discover How We Can Help - Request a Consultation
X-linked Duchenne Muscular Dystrophy (DMD)
Duchenne Muscular Dystrophy is a severe, progressive genetic disorder caused by mutations in the DMD gene, which result in the absence of the dystrophin protein. Dystrophin is crucial for maintaining the structural integrity of muscle fibers. The loss of this protein leads to chronic muscle damage, inflammation, and eventual replacement by fibrotic and adipose tissue. This foundational understanding is critical for developing targeted gene therapies that address the root cause of the disease.
Pathogenesis
DMD pathogenesis is tied to functional dystrophin deficiency. Dystrophin, a key part of the sarcolemma's dystrophin-glycoprotein complex (DGC), links actin cytoskeleton to extracellular matrix. Its absence makes muscle fibers prone to contraction damage, triggering degeneration-regeneration cycles. Exhausted muscle stem cell regenerative capacity leads to muscle necrosis, scar/fat replacement, and progressive function loss.
Therapeutic Targets
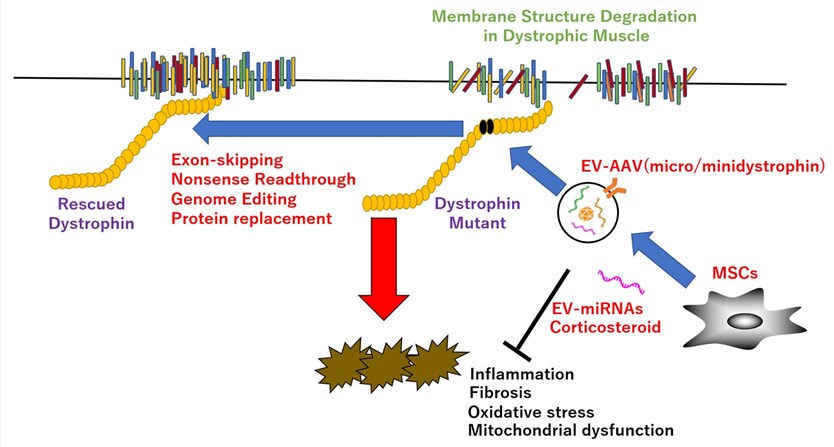 Fig.1 Treatment strategies for DMD.1
Fig.1 Treatment strategies for DMD.1
Focused on addressing the root cause (monogenic mutation) to restore functional dystrophin, via two main approaches:
- Exon Skipping: Antisense oligonucleotides restore DMD gene reading frame for truncated but functional dystrophin.
- Gene Therapy: Deliver functional dystrophin gene to muscle cells.
Treatment Methods Related to AAV
AAV is the leading DMD gene therapy delivery vector, enabling efficient muscle transduction and long-term expression. Due to full-length DMD gene size, "micro-dystrophin" (miniaturized, functional version) is used for AAV packaging. This single-dose approach shows promise, but host immune reactions to vectors/transgenes remain a key challenge limiting efficacy.
Workflow
- Required Starting Materials: Clients provide a detailed project proposal, including targeted DMD mutation profile, existing vector designs/data, and specific therapeutic goals.
- Initial Consultation & Project Scoping: Deep dive into the project to define therapeutic outcomes, target tissues, and key deliverables for alignment.
- Vector & Transgene Design: Custom AAV serotype design for optimal delivery; development of functional "micro-dystrophin" to fit vector packaging capacity.
- Vector Construction & Production: State-of-the-art facilities produce high-purity, high-titer custom rAAV vectors.
- Preclinical Validation (In Vitro & In Vivo): Validate vector expression in cell models; assess efficacy and safety in animal models.
- Data Analysis & Final Report: Compile raw/analyzed data into a comprehensive report with findings and clinical program roadmap.
- Final Deliverables: Ready-to-use AAV vector, scientific report (preclinical data), and project findings summary.
- Estimated Timeframe: 8-16 weeks, variable based on design complexity and in vivo testing scope.
What we can offer
Our Advantage
One-Stop Solution
Creative Biolabs offers end-to-end service from vector design/optimization to preclinical validation, saving time and resources.
Efficient Process Development
Streamlined vector and transgene design processes accelerate progression from concept to validated candidate.
Scalable Production
Scalable, high-titer rAAV production caters to needs from small-scale validation to large preclinical studies.
Quality-by-Design (QbD)
Adheres to QbD and process analytical frameworks for meticulous, quality-focused process execution.
Aseptic Procedures
Strict aseptic verification throughout production ensures final product sterility and safety.
GMP-Ready
Production aligns with GMP principles, enabling smooth transition to clinical trials.
Customization and Optimization
Fully customized service, including codon optimization for max expression and engineering for higher therapeutic yield.
Rigorous Quality Control
High-standard QC tools quantify and assess rAAV quality for consistent, reliable results.
Experience the Creative Biolabs Advantage - Get a Quote Today
Customer Reviews

FAQs
For the muscle-targeting needs of DMD (especially cardiac muscle), what core data supports Creative Biolabs' selection of AAV serotypes and micro-dystrophin sequences? Can adjustments and optimizations be made based on our animal models (e.g., mdx mice)?
Serotypes (e.g., AAV9, AAVrh74) are selected based on preclinical data regarding their transduction efficiency and long-term retention in cardiac and skeletal muscles. The micro-dystrophin sequence retains core functional domains and is modified to enhance expression stability. Adjustments to serotypes and sequence details can be made according to specific animal models.
The immunogenicity of AAV vectors is a core bottleneck in DMD clinical translation. What specific measures does Creative Biolabs take to reduce neutralizing antibodies and T-cell responses triggered by capsids? Can preclinical immunogenicity assessment data be provided?
Immunogenicity is reduced through capsid mutation modification and production process optimization. Preclinical assessment data (e.g., antibody titers, inflammatory factor levels in animal models) can be provided. Additionally, vector design is optimized to minimize the risk of immunity-mediated expression silencing.
During the scale-up from research-grade to preclinical-grade production, how does Creative Biolabs ensure the titer stability, empty capsid control (target <10%), and purity compliance of rAAV vectors? Do the vectors meet FDA/EMA preclinical material standards?
A HEK293 suspension/adherent production system is used, and impurities are removed via standardized chromatographic purification. Consistency data from at least 3 consecutive batches is provided. The final product has host protein residues <10ng/mL and endotoxin <0.1EU/mL, fully complying with international regulatory standards.
Creative Biolabs leads in next-gen DMD gene therapies. Our services, from custom rAAV design to full preclinical validation, accelerate research and speed life-changing treatments to patients. Combining our expertise with your vision, we aim to overcome DMD therapy challenges and redefine its future.
Contact Our Team for More Information and to Discuss Your Project
Reference
- Matsuzaka, Yasunari, et al. "Therapeutic application of extracellular vesicles-capsulated adeno-associated virus vector via nSMase2/Smpd3, satellite, and immune cells in Duchenne muscular dystrophy." International Journal of Molecular Sciences 23.3 (2022): 1551. https://doi.org/10.3390/ijms23031551. Distributed under Open Access license CC BY 4.0, without modification.
