Development Service of AAV Vector for Heart Failure Research
Heart failure remains a formidable challenge in cardiovascular medicine, characterized by complex molecular pathogenesis and limited regenerative capacity of the adult myocardium. Gene therapy utilizing Adeno-associated Virus (AAV) vectors has emerged as a transformative strategic approach, offering the potential to directly correct underlying molecular defects, enhance cardiac contractility, and promote functional recovery. The unique tropism of specific AAV serotypes for cardiac tissue, particularly cardiomyocytes, enables targeted gene delivery with minimal off-target effects, making it an ideal platform for addressing the intricate pathophysiology of heart failure.
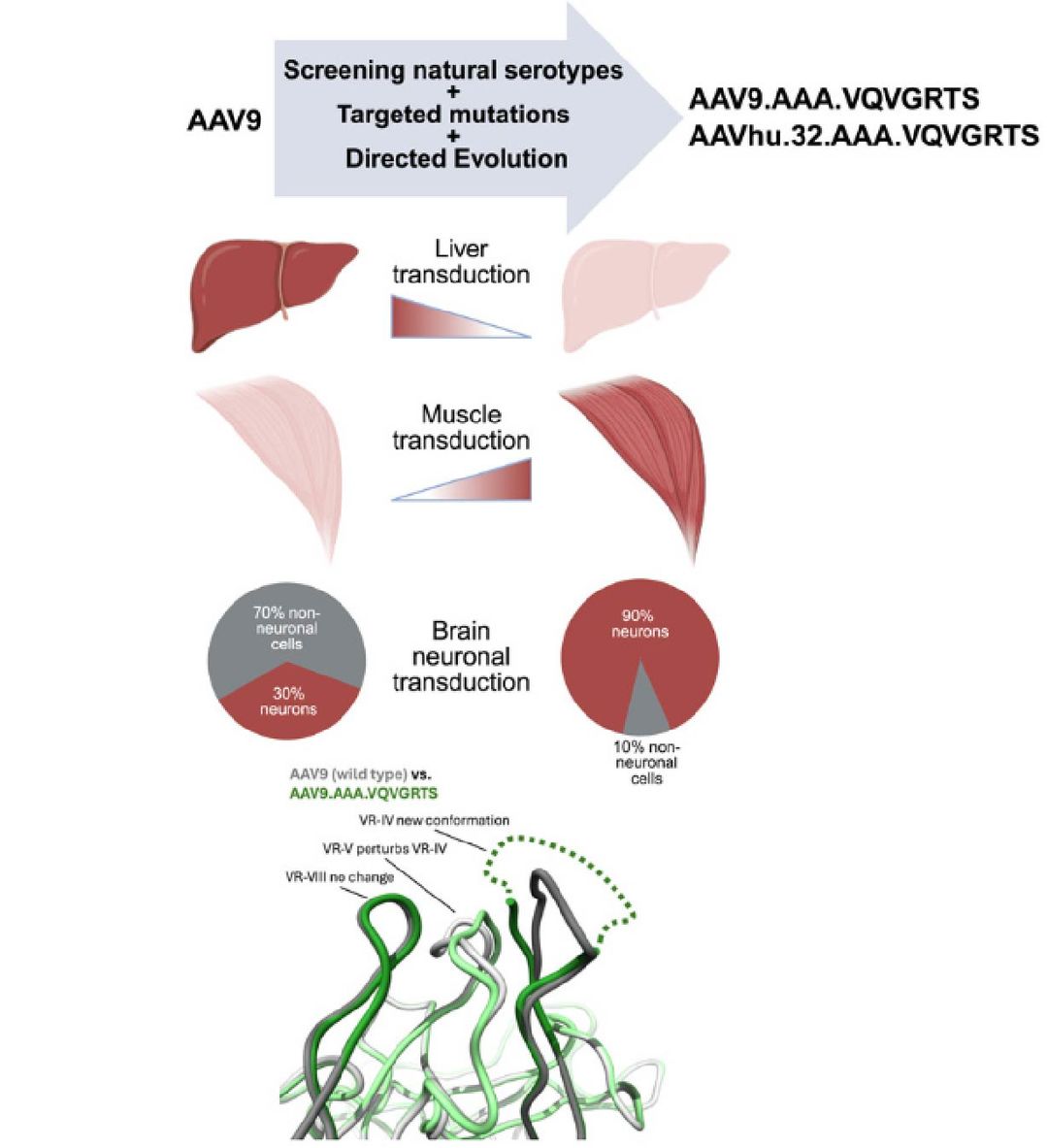 Fig.1 Directed evolution of liver-detargeted AAV vectors for systemic gene delivery to skeletal muscle and heart 1,2
Fig.1 Directed evolution of liver-detargeted AAV vectors for systemic gene delivery to skeletal muscle and heart 1,2
Our Development Service of AAV Vector for Heart Failure Research is a specialized, end-to-end solution designed to empower your pre-clinical investigations. We combine deep cardiovascular biology expertise with state-of-the-art virology to engineer and produce precision AAV vectors that deliver your therapeutic transgene efficiently and specifically to the heart, accelerating the translation of novel therapeutic concepts into tangible pre-clinical evidence.
Our Comprehensive Service Workflow for Heart Failure Research
Our process is meticulously tailored to address the specific demands of cardiac gene therapy, from vector conceptualization to the delivery of a research-ready, heart-tropic AAV product.
Cardiovascular-Focused Consultation: We initiate a collaborative dialogue to understand your specific hypothesis—whether it involves enhancing calcium handling, modulating β-adrenergic signaling, inhibiting apoptosis, or promoting angiogenesis. We then recommend the optimal strategic combination of promoter, transgene, and capsid.
Cardiac-Optimized Vector Design: Our expertise is applied to designing a highly efficient expression cassette. This includes selecting from a panel of cardiac-specific promoters (e.g., cTnT, MHC, MLC-2v) to restrict expression predominantly to cardiomyocytes, and codon-optimizing your Gene of Interest (GOI) for robust protein expression in the cardiac cellular environment.
Precise Vector Construction & Validation: Using advanced cloning techniques, we assemble the final construct in an AAV plasmid backbone. The integrity of the entire cassette, including the cardiac promoter and the therapeutic transgene, is unequivocally confirmed through 100% Sanger sequencing.
Production of Cardio-Tropic AAV Serotypes: We produce the AAV vector using the robust triple-transfection method in HEK293 cells, specializing in serotypes with proven cardiac tropism for pre-clinical models, such as AAV9, AAV8, AAV6, and novel engineered capsids (e.g., AAV2i8, AAV-DJ).
High-Purity Purification for In Vivo Studies: The crude viral harvest is purified using iodixanol gradient ultracentrifugation or affinity chromatography. This critical step enriches for fully packaged genomes and removes empty capsids, a key factor in reducing non-specific immune activation and maximizing the effective dose delivered to the heart.
Rigorous In Vitro Functional Screening : We can perform preliminary functional validation in relevant cardiac cell lines (e.g., H9c2, AC16) or induced pluripotent stem cell-derived cardiomyocytes (iPSC-CMs) to confirm transgene expression and preliminary biological activity before proceeding to animal studies.
Comprehensive QC & Formulation for Animal Dosing: The final product is thoroughly characterized for genomic titer (ddPCR/qPCR), purity (SDS-PAGE/SEC-HPLC), and endotoxin levels. It is formulated in a sterile, biocompatible buffer suitable for systemic or direct intramyocardial delivery in rodent or large animal models.
Core Service Highlights for Cardiac Applications
| Service Phase | Key Activities & Cardiac-Specific Value |
|---|---|
| Strategic Design | Selection of cardiac-specific/specific promoters (cTnT, MHC) and cardio-tropic serotypes (AAV9, AAV8) to maximize on-target expression and minimize off-target effects. |
| Vector Engineering | Codon optimization for cardiomyocytes; expertise in cloning challenging cardiac targets (e.g., SERCA2a, S100A1, Parvalbumin, FGFs). |
| High-Fidelity Production | Scalable production of high-titer, high-purity AAV vectors with optimized full-to-empty capsid ratios, crucial for dose-dependent efficacy in heart failure models. |
| Quality & Safety Analytics | Comprehensive profiling including titer, capsid purity, sterility, and low-endotoxin certification, ensuring safety for sensitive in vivo cardiac function studies. |
Our Distinct Advantages for Heart Failure Research
Deep-Seated Cardiovascular Molecular Expertise: Our team includes scientists with direct experience in cardiac pathophysiology, enabling us to provide insightful guidance on targeting key pathways involved in heart failure, such as calcium cycling, energetics, hypertrophy, and fibrosis.
Validated Toolkit for Cardiac Targeting: We offer a curated portfolio of well-characterized, cardiac-restricted promoters and the most efficient natural and engineered AAV capsids for robust and specific transgene expression in the myocardium of small and large animal models.
Focus on Product Potency and Purity: We prioritize the delivery of AAV preparations with a high percentage of full capsids. This ensures that a greater proportion of the administered dose delivers the therapeutic gene, enhancing efficacy and reducing the total viral load required—a critical consideration for minimizing immunogenicity in chronic heart failure studies.
Integrated In Vitro Screening Capability: Our optional in vitro functional assessment in relevant cardiac cells provides an invaluable preliminary data point, de-risking your project and informing dosing strategy before you commit to more resource-intensive animal experiments.
A Collaborative Partnership Model: We function as an extension of your research team. From designing a vector that answers your specific biological question to providing the comprehensive CofA necessary for accurate animal dosing, we are committed to the scientific rigor and success of your heart failure gene therapy program.
What Our Clients Say
"Our research focuses on restoring SERCA2a function in a model of heart failure with preserved ejection fraction (HFpEF). The team's knowledge of cardiotropic AAVs was exceptional. They guided us to a novel engineered capsid that showed superior cardiac uptake over AAV9 in our pilot study. The vector purity was outstanding, and the high infectious titer allowed us to use a minimal, safer dose for our chronic study."
— Dr. Rebecca Harris
"As a biotech startup developing a gene therapy for dilated cardiomyopathy, we needed a CDMO partner that understood both vector development and the regulatory path. Their comprehensive service, from codon-optimizing our proprietary transgene to delivering a GMP-like pre-clinical batch with exhaustive documentation, has been invaluable. Their proactive approach to optimizing the full/empty ratio directly impacted our efficacy results."
— Dr. Mark Jenkins
"We required a dual-vector system to express a large therapeutic protein. The complexity was significant, but their molecular biology team engineered a flawless solution. The vectors performed perfectly in our murine ischemia-reperfusion model, showing coordinated expression specifically in the infarct border zone. The quality of the data we obtained accelerated our publication timeline considerably."
— Professor Sarah Goldman
FAQ
Q: Which AAV serotype and promoter are most effective for heart failure research?
AAV9 is widely regarded as the gold standard for robust cardiac transduction in rodents and larger animals via systemic administration. AAV8 and AAV6 also show strong cardio-tropism. For promoters, the cardiac Troponin T (cTnT) promoter offers high specificity for cardiomyocytes, while the α-Myosin Heavy Chain (α-MHC) promoter is also highly cardiac-specific, though its activity can be species- and condition-dependent. We will advise on the optimal combination for your specific model and goals.
Q: What are the key considerations for dosing AAV in heart failure models?
Key factors include the route of administration (systemic vs. direct intramyocardial), the model species (mouse, rat, porcine), the disease stage, and the desired level and duration of expression. Accurate dosing relies on knowing the genomic titer (vg/kg) precisely. The purity of the preparation (high full/empty ratio) is critical, as empty capsids can sequester neutralizing antibodies and contribute to inflammation without therapeutic benefit.
Q: How can handle the cloning and production of large or complex cardiac transgenes?
Absolutely. We have extensive experience in handling challenging genetic elements common in cardiovascular research, including large cDNA sequences (e.g., for dystrophin minigenes) and complex regulatory systems. Our homologous recombination-based cloning is ideal for assembling large constructs without introducing unwanted restriction sites.
Q: How do you ensure the AAV vector is safe for use in sensitive animal models of heart failure?
- Endotoxin Testing: Quantified to ensure levels are below the threshold known to provoke an inflammatory response, which is crucial in compromised hearts.
- Sterility Testing: Confirmation of the absence of microbial contamination.
- Mycoplasma Testing: A sensitive PCR-based assay to rule out this common cell culture contaminant.
- Purity Analysis: SDS-PAGE and HPLC analysis to confirm capsid protein integrity and the absence of significant host cell protein contaminants.
Q: What deliverables will I receive for my pre-clinical study?
- The Ready-to-Use AAV Vector: A high-titer, purified AAV stock, formulated and aliquoted for your in vivo studies.
- Detailed Certificate of Analysis (CoA): Documents the genomic titer (vg/mL), functional titer (if applicable), serotype, promoter, purification method, endotoxin level, and results of all purity and safety tests.
- Comprehensive Project Report: A full account of the vector design, construction, production, and QC process.
Conclusion
The promise of gene therapy for heart failure hinges on the precise and efficient delivery of therapeutic genes to the struggling myocardium. Our Development Service of AAV Vector for Heart Failure Research is specifically engineered to fulfill this promise at the pre-clinical stage. By integrating deep cardiovascular expertise with cutting-edge virology, we provide you with a powerful, validated, and reliable tool that translates your scientific vision into a targeted therapeutic intervention. Partner with us to ensure that your gene therapy construct is not just another reagent, but a scientifically robust and strategically engineered key to unlocking new insights and potential treatments for heart failure.
References
- Elad Firnberg, et al. "Directed evolution of liver-detargeted AAV vectors for systemic gene delivery to skeletal muscle and heart" Volume 33 (2025), Issue 4101571 https://doi.org/10.1016/j.omtm.2025.101571
- Distributed under Open Access license CC BY 4.0, without modification.
