Oncolytic Measles Virus Construction Service
Measles virus (MV) is frequently utilized as an oncolytic virus for infecting and lysing tumor cells. It is characterized by its tumor-tropic property, enabling it to bind to specific receptors on tumor cells, thereby eliminating tumors while sparing normal cells. Leveraging years of expertise, Creative Biolabs has constructed an oncolytic virus engineering platform. This platform is capable of offering design, editing, construction, and production services for oncolytic measles virus tailored to clients' requirements.
Introduction of Measles Virus
MV is a negative-strand RNA virus belonging to the genus Morbillivirus within the family Paramyxoviridae. Its non-segmented genome is approximately 16 kb in length and encodes six structural and two non-structural proteins. The viral glycoproteins, hemagglutinin (H), and fusion (F) proteins mediate receptor binding and membrane fusion at the plasma membrane, respectively. The virus is enveloped by a membrane containing two viral glycoproteins, H and F. The H protein mediates attachment to host-cell receptors, while the F protein directs the viral envelope to fuse with the host plasma membrane, resulting in syncytium formation.
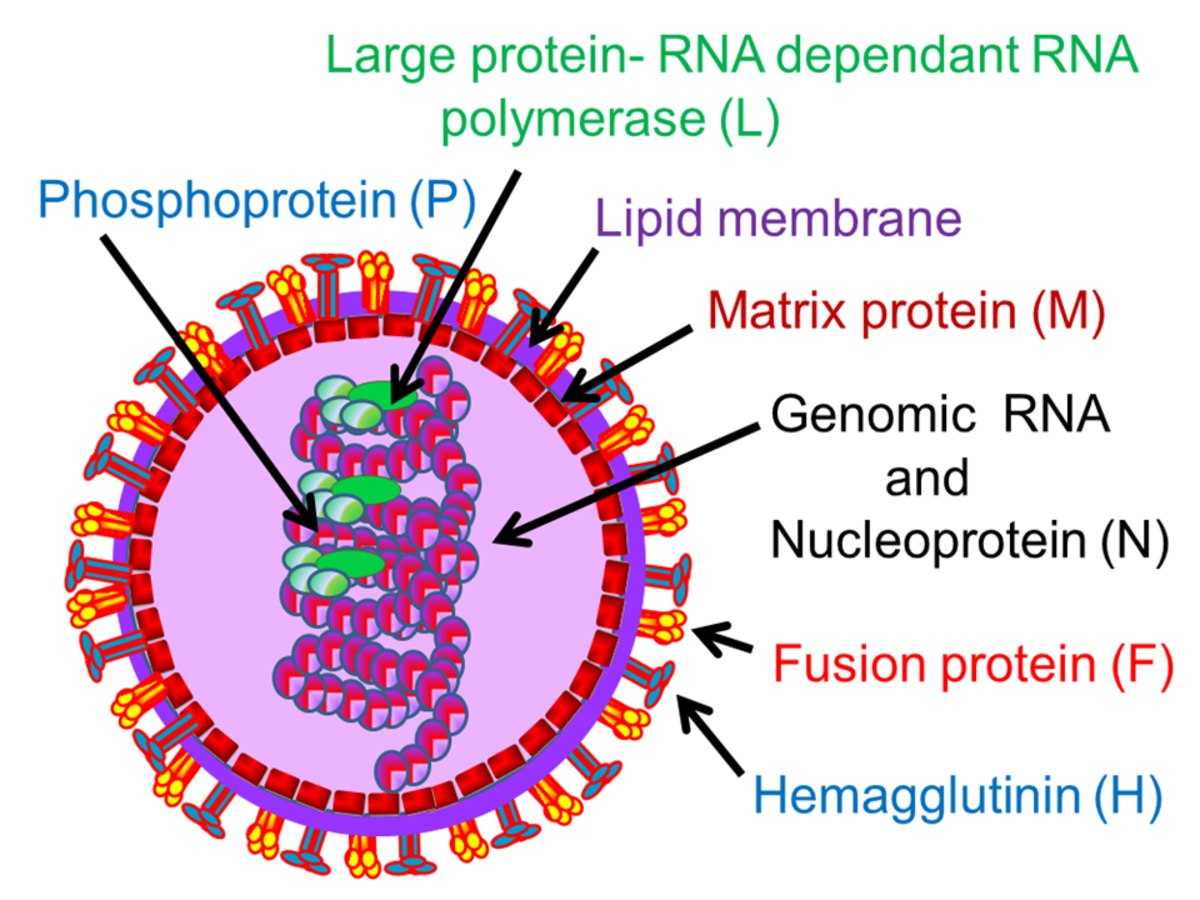 Fig.1 The structure of the measles virus.Distributed under CC BY-SA 4.0, from Wiki, without modification.
Fig.1 The structure of the measles virus.Distributed under CC BY-SA 4.0, from Wiki, without modification.
The MV genome encodes several proteins, including nucleoprotein (N), phosphoprotein (P), matrix (M), fusion protein (F), hemagglutinin (H, also known as the spike protein), and polymerase (L). Notably, the P gene gives rise to three distinct proteins, namely P, C, and V, while each of the remaining genes encodes a single protein.
![]() Fig.2 The open reading frame of the measles virus genome encodes six structural proteins.1
Fig.2 The open reading frame of the measles virus genome encodes six structural proteins.1
The receptor of MV:
- Signaling lymphocyte activation molecule (SLAM) is predominantly expressed on the surfaces of activated T lymphocytes, B lymphocytes, dendritic cells, monocytes, and natural killer cells. The H protein of the MV attaches to SLAM. This attachment initiates the merging of the viral envelope with the cell membrane, thereby allowing the virus to penetrate the cell.
- Cell adhesion molecule nectin-4, a member of the nectin family, is a transmembrane protein. It is highly expressed on the surfaces of respiratory epithelial cells and certain tumor cells. Multiple domains within its extracellular region are capable of interacting with the H protein of the measles virus.
- CD46, a complement-regulatory protein, is ubiquitously expressed on the surfaces of human cells. It contains multiple short consensus repeat (SCR) domains. The measles virus exploits CD46 to gain entry into cells via endocytosis.
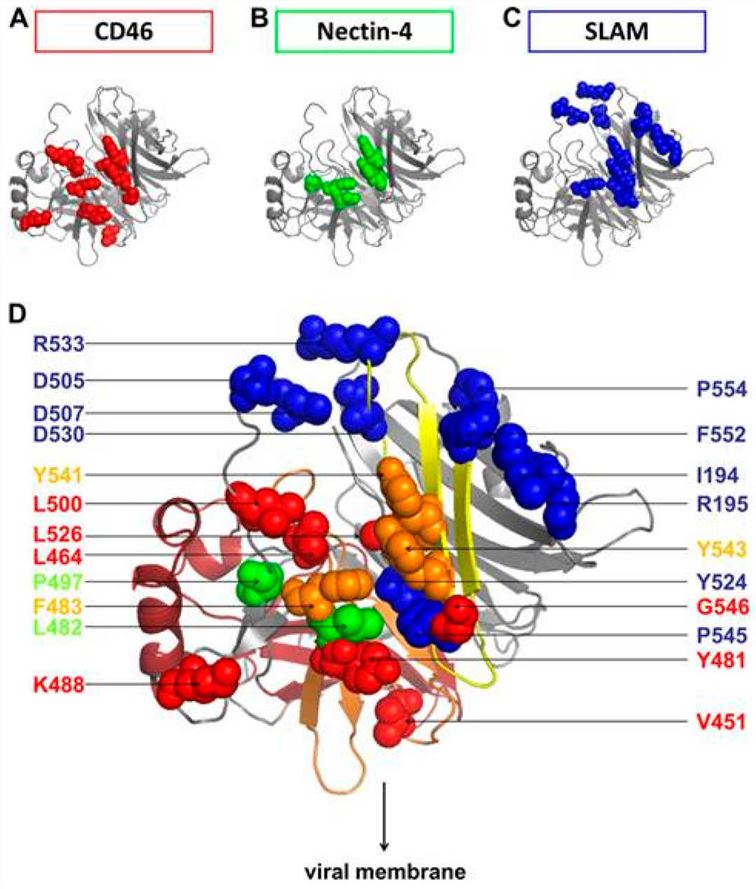 Fig.3 The major residues in the head region of the H protein are responsible for binding to the receptor.2
Fig.3 The major residues in the head region of the H protein are responsible for binding to the receptor.2
Genetic Modifications of Oncolytic VSV Vectors
- Insert reporter gene
Insertion of reporter genes enables the tracking of MV replication. The introduction of genes related to carcinoembryonic antigen (CEA) and β-human chorionic gonadotropin (HCG) facilitates the detection of MV in routine clinical laboratory settings. When MV encodes the sodium-iodide symporter NIS, the resulting MV-NIS can utilize iodine-123 for γ-camera imaging. Recently, recombinant MeV variants encoding fluorescent reporter genes, with the aid of two-photon microscopy, have achieved in vivo imaging of virus transmission at single-cell resolution, which contributes to in-depth research on MV.
- Retargeting MeV
Retargeting of MV can be achieved by altering the intrinsic receptor-binding site and fusing the antibody scFv to the C-terminus of the viral hemagglutinin. For instance, oncolytic MeV can be targeted to the myeloma surface antigen CD38, B-cell malignancies via targeting CD20, ovarian cancer by targeting the folate receptor (FR)-α, and glioblastoma expressing EGFRⅧ.
- Immunovirotherapy
Oncolytic MV exerts pleiotropic effects on anti-tumor immune responses. It induces the generation of a unique set of immune peptides and promotes the activation of related immune cells to target tumors.
Tab.1 Immunomodulatory oncolytic MV.
| Immunomodulator | Anticipated Immunological Effects | Preclinical Data |
|---|---|---|
| GM-CSF | Promote the activation and maturation of DC, monocytes, macrophages, neutrophils, and NK cells. | Superior anti-tumor efficacy |
| Promote the infiltration of neutrophils and T cells | ||
| Enhanced tumor-specific T cell responses | ||
| INF-β | Enhanced antitumor response via innate and adaptive effector mechanisms | Increased the infiltration of CD68+ macrophages |
| Improved survival | ||
| α-PD-L1/α-CTLA | Enhanced the antitumor T cell response | Delayed tumor progression |
| Prolonged survival | ||
| Increased tumor-specific IFN-γ response | ||
| IL-12/IL-15 | Activation and recruitment of T cells and NK cells | Increased expression of effector cytokines |
| T-cell infiltration | ||
| Tumor-specific IFN-γ expression | ||
| Bispecific antibody | Recruitment of T cells, enhanced T cell antitumor cytotoxicity | Increased T cell infiltration |
| Induce tumor-specific immunity | ||
| TAA | Priming and activation of TAA-specific T cells | Priming and activation of TAA-specific T cells |
Featured Services and Construction Workflow
We provide a comprehensive range of MV services based on our advanced OncoVirapy™ platform, including but not limited to the following items:
- Select the GOI (encoding antibodies, cytokines, chemokines or immune checkpoint inhibitors, etc.) or the genes plan to be deleted for restriction mapping and sequencing verification
- Functional validation of transgenes
- Expression cassette design with the most appropriate promoter to guarantee the high-level expression
- Viral genes engineering (single gene or different genes combination)
- Different construction strategies (homologous recombination, BAC system, etc.)
- MV amplification, purification, titering services
- MV whole genome sequencing (WGS) service
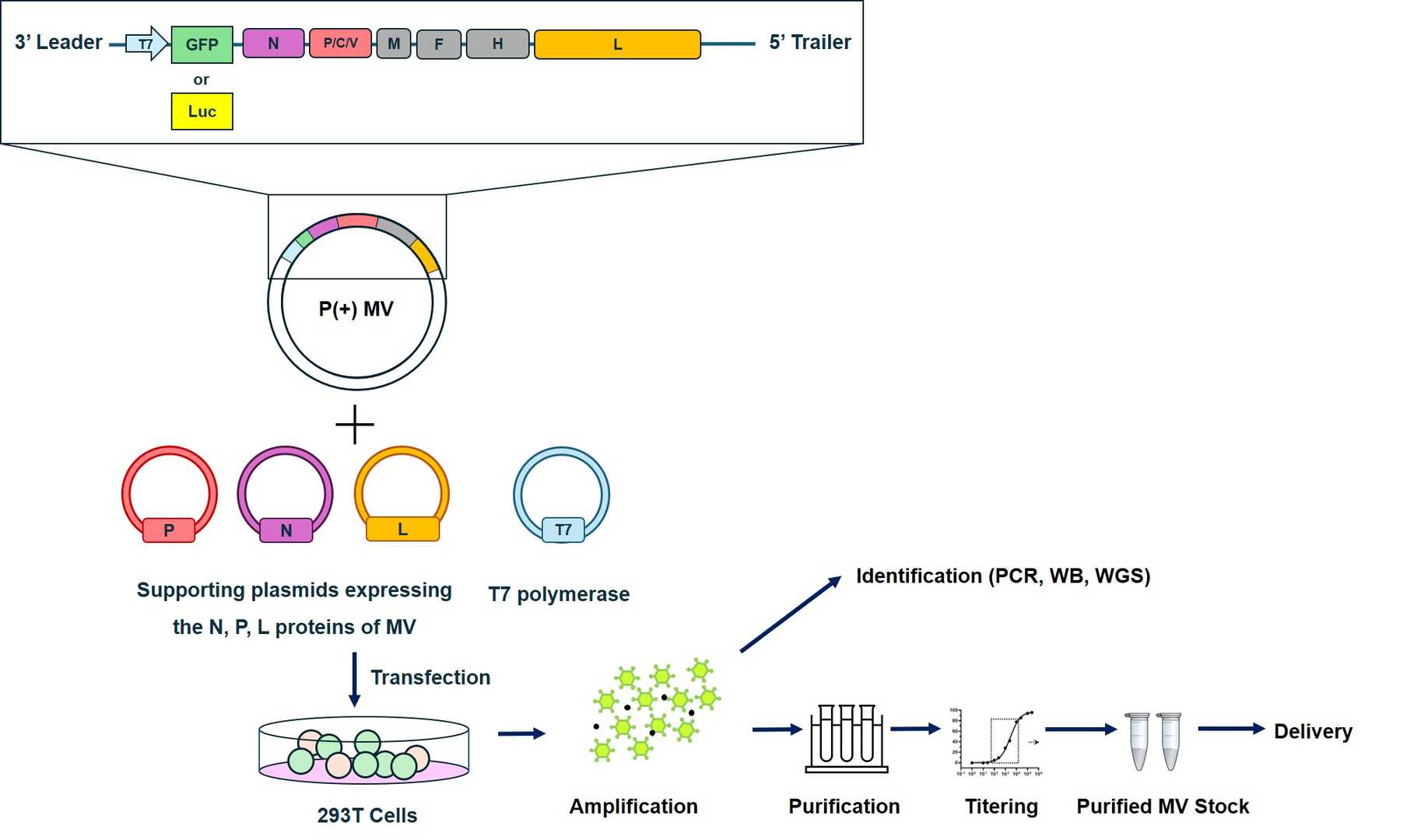 Fig.4 Workflow for Creative Biolabs construction of oncolytic Measles virus.
Fig.4 Workflow for Creative Biolabs construction of oncolytic Measles virus.
Cases of VSV Genetic Modifications
Tab.2 Oncolytic measles virus for pre-clinical trials.
| MV Strain | Genetic Modification | Type of Cancer | Route of Administration |
|---|---|---|---|
| MV-EZ | Genetically unmodified | CTLC | Intratumoral |
| MV-CEA | Engineered to express the soluble extracellular domain of human CEA | Ovarian cancer | Intraperitoneal |
| Glioblastoma multiforme | Intracranial | ||
| MV-NIS | Engineered to express NIS | Ovarian cancer | Intraperitoneal |
| Multiple myeloma | Intravenous | ||
| Mesothelioma | Intravenous | ||
| Malignant Peripheral nerve sheath tumors | Intratumorally |
Creative Biolabs offers an array of tailored gene-editing services for the oncolytic measles virus. These services are characterized by their high-level accuracy and all-encompassing technical assistance. Additionally, we present pre-clinical assessment services (either in vivo or in vitro) for the modified oncolytic agents, aiming to expedite the client's research advancement.
References
- Guseva, Serafima, et al. "The nucleoprotein and phosphoprotein of measles virus." Frontiers in microbiology 10 (2019): 1832. Distributed under Open Access license CC BY 4.0, using only Part A of the original image.
- Lin, Liang-Tzung, and Christopher D. Richardson. "The host cell receptors for measles virus and their interaction with the viral hemagglutinin (H) protein." Viruses 8.9 (2016): 250. Distributed under Open Access license CC BY 4.0, without modification.
