Cancer Vaccine & Oncolytic Virotherapy Combination Therapy Development Service
Accelerate Your Cancer Immunotherapy Development Process!
How Creative Biolabs Can Assist Your Project?
Many researchers encounter long development cycles and challenges in developing effective cancer combination therapies. Creative Biolabs' Oncolytic Virotherapy Development for Combination Therapy with Cancer Vaccines addresses these issues by offering tailored solutions and expertise in oncolytic virus-based strategies to accelerate cancer immunotherapy development. The company provides comprehensive services to support oncolytic virotherapy projects, delivering customized approaches to help clients effectively integrate oncolytic viruses (OVs) with cancer vaccines, and offers the framework and tools needed to design, construct, and validate oncolytic viruses for combination therapy.
Workflow
| Required Starting Materials | Project Consultation and Design |
|---|---|
|
Professional technicians actively communicate with clients to understand the project goals, target antigens of interest, and oncolytic viruses. This will be followed by a comprehensive project proposal as well as an OV construction strategy, experimental plan, expected completion time, and deliverables. |
| OV Vector Construction | In Vitro OV Characterization |
| Creative Biolabs employs advanced recombinant DNA technology to design OVs that express target TAA or enhance vaccine efficacy, potentially involving modification of the viral genome to improve tumor selectivity, enhance immunogenicity, or incorporate therapeutic genes. | The constructed OVs undergo rigorous in vitro testing to assess their replication efficiency, tumor cell selectivity, and cytotoxic activity. We also evaluate the expression of transgene products and confirm the genetic stability of the OVs. |
| In Vivo Preclinical Studies | Data Analysis and Reporting |
| Creative Biolabs conducts preclinical studies in relevant animal models to evaluate the safety, efficacy, and immunogenicity of the OV-vaccine combination therapy. This includes assessing tumor growth inhibition, immune response induction, and potential toxicity. | We provide detailed reports summarizing the experimental procedures, results, and statistical analyses. Our team of experts will work closely with you to interpret the data and discuss potential next steps. |
| Final Deliverables | Estimated Timeframe |
|
Detailed study reports with raw and analyzed data. Characterized OV constructs (e.g., purified virus stocks). Optimized protocols for OV production and application. |
The typical timeframe for this service is 12 to 20 weeks, depending on the oncolytic virus design complexity, in vivo model requirements, and study scope. |
[Discover How We Can Help - Request a Consultation]
Case Study
The implementation of combination therapy incorporating engineered oncolytic viruses and cancer vaccines in standard in vivo murine models and in vitro neoplastic cell line systems, particularly those for ovarian cancer, has led to a notable boost in anti-tumor effectiveness. Outcomes from a plethora of published research projects offer important outlooks on its substantial prospects for treating ovarian cancer.
| Cytotoxicity | Virus transfection |
|---|---|
|
|
|
| Extracellular ATP assay | Flow Cytometry |
|
|
|
| Tumor Volume | |
|
|
|
Customer Reviews
-
"Using Creative Biolabs' oncolytic virotherapy development services in our research has significantly accelerated our ability to evaluate novel combination immunotherapy strategies."
J**n L***[6 Months]
-
"Creative Biolabs' expertise in OV engineering and preclinical modeling was crucial in facilitating the development of our next-generation cancer vaccine."
M***a S***[9 Months]
-
"Creative Biolabs' comprehensive data analysis and reporting have significantly improved the clarity and impact of our publications."
D***d K***[12 Months]
[Experience the Creative Biolabs Advantage-Get a Quote Today]
What We Can Offer
- Customized Solutions: We understand that each project is unique. Creative Biolabs provides tailored solutions to meet your specific research needs, ensuring that our oncolytic virotherapy strategies align perfectly with your project goals.
- Expertise in OV Engineering: Our team possesses extensive knowledge in oncolytic virus engineering, allowing for the precise design and construction of OVs to express your target tumor-associated antigens (TAAs) or enhance vaccine efficacy.
- Advanced Vector Construction: Creative Biolabs employs cutting-edge recombinant DNA technologies to engineer OVs. This includes modifying viral genomes to improve tumor selectivity, enhance immunogenicity, or incorporate therapeutic genes, ensuring the generation of OV vectors with desired genetic modifications.
- Rigorous In Vitro Characterization: We conduct thorough in vitro testing to assess the replication efficiency, tumor cell selectivity, and cytotoxic activity of your constructed OVs. We also evaluate transgene product expression and confirm the genetic stability of the OVs.
- Comprehensive Preclinical Studies: Creative Biolabs performs in vivo preclinical studies in relevant animal models. These studies evaluate the safety, efficacy, and immunogenicity of OV-vaccine combination therapies, providing crucial data on tumor growth inhibition, immune response induction, and potential toxicity.
- Detailed Data Analysis and Reporting: We deliver detailed reports summarizing experimental procedures, results, and statistical analyses. Our experts work closely with you to interpret data and discuss potential next steps, ensuring clarity and actionable insights.
- High-Quality Deliverables: Clients receive a comprehensive suite of deliverables, including detailed study reports, characterized OV constructs, and optimized protocols for OV production and application.
- Project Consultation and Design: We initiate the process with a thorough consultation to grasp your project objectives, target antigens, and the specific traits you desire for OVs. Based on this understanding, we will formulate a tailor-made strategy for the construction and refinement of OVs.
Why Choose Us?
Creative Biolabs is a trusted partner for oncolytic virotherapy development, offering several key advantages:
- Expertise: Creative Biolabs has extensive experience in oncolytic virus engineering, preclinical research, and cancer immunotherapy. Our team comprises experts in virology, immunology, and molecular biology.
- Customization: We provide tailored solutions to meet your specific research needs. We work closely with you to design and execute experiments that address your unique challenges.
- Advanced Technology: Creative Biolabs utilizes cutting-edge technologies and platforms to ensure the highest quality and efficiency in OV development.
- Comprehensive Services: From OV design and construction to preclinical evaluation, we offer a full range of services to support your project from start to finish.
- Published Data: Creative Biolabs is committed to delivering reliable and reproducible results.
[Experience the Creative Biolabs Advantage - Get a Quote Today]
Introduction
Oncolytic virotherapy is a promising approach in cancer treatment, utilizing viruses to selectively target and destroy tumor cells. The field has witnessed significant advancements, with ongoing research focusing on enhancing OV efficacy and safety. Combination therapies, particularly those involving cancer vaccines, are emerging as a powerful strategy to harness the full potential of oncolytic viruses and stimulate durable anti-tumor immunity.
Oncolytic Virotherapy Development for Combination Therapy with Cancer Vaccines
Oncolytic viruses can be used as viral vectors to deliver therapeutic genes, including those encoding tumor-associated antigens, directly to tumor cells. This approach enhances the immunogenicity of cancer vaccines by promoting antigen presentation and stimulating potent anti-tumor immune responses. Furthermore, the combination of oncolytic virotherapy with cancer vaccines can create a synergistic effect, where the OV-mediated tumor lysis releases tumor antigens, further amplifying the vaccine-induced immune response.
Tab.1 TAA encoding OV for cancer vaccination.
| Oncolytic Virus | Tumor Antigen | Tumor model |
|---|---|---|
| Adenovirus | DCT | B16F10 |
| gp33 | ||
| MART-1 | NSFA-MART-1 | |
| PSA/PSCA | RM11-PSA | |
| HSV | PAP | TRAMP-C2 |
| Vaccine Virus | CEA | MC38-CEA |
| E6/E7 | TC-1 | |
| gp33 | B16F10 | |
| HY | MB49 | |
| Ova | MOSE-Ova | |
| VSV | BRAF | B16F10 |
| E6/E7 | TC-1 | |
| Ova | B16F10-Ova | |
| gp-100 | ||
| gp33 |
Creative Biolabs is dedicated to providing cutting-edge solutions for oncolytic virotherapy development, particularly in the context of combination therapy with cancer vaccines. Our services are designed to empower researchers and biopharmaceutical companies in their quest to develop more effective cancer immunotherapies. If you have any need, please feel free to contact us!
Reference
- Mathlouthi, Sara, et al. "Extracellular vesicles powered cancer immunotherapy: Targeted delivery of adenovirus-based cancer vaccine in humanized melanoma model." Journal of Controlled Release 376 (2024): 777-793. DOI: 10.1016/j.jconrel.2024.10.057. Distributed under Open Access license CC BY 4.0, Some of the pictures were edited and reformatted.

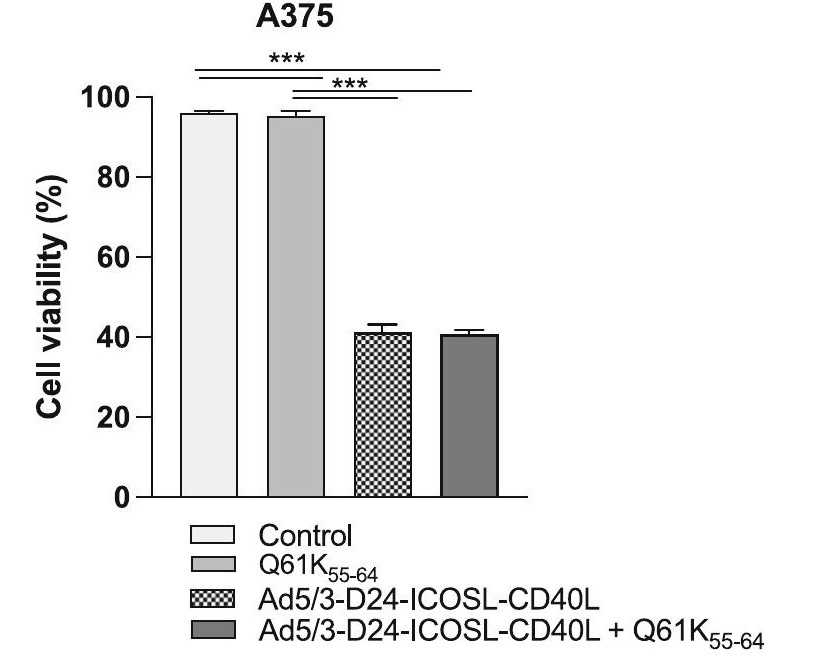 Fig.1 Cell viability is assessed by MTS assay.1
Fig.1 Cell viability is assessed by MTS assay.1
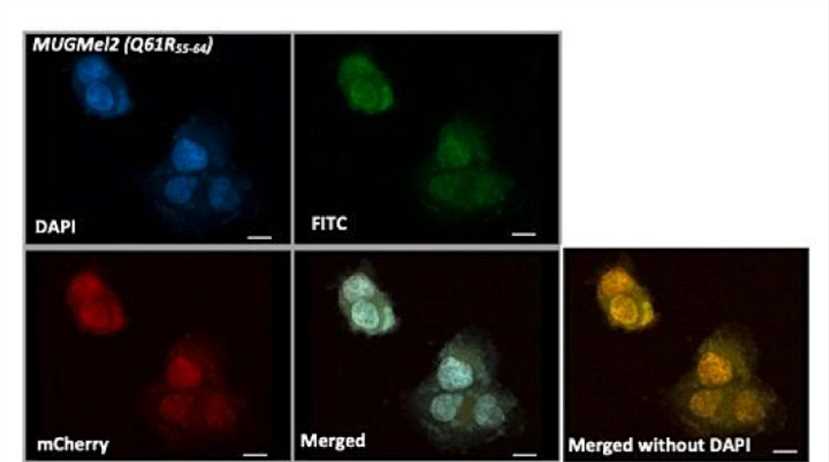 Fig.2 Transduction efficiency is assessed by confocal microscopy.1
Fig.2 Transduction efficiency is assessed by confocal microscopy.1
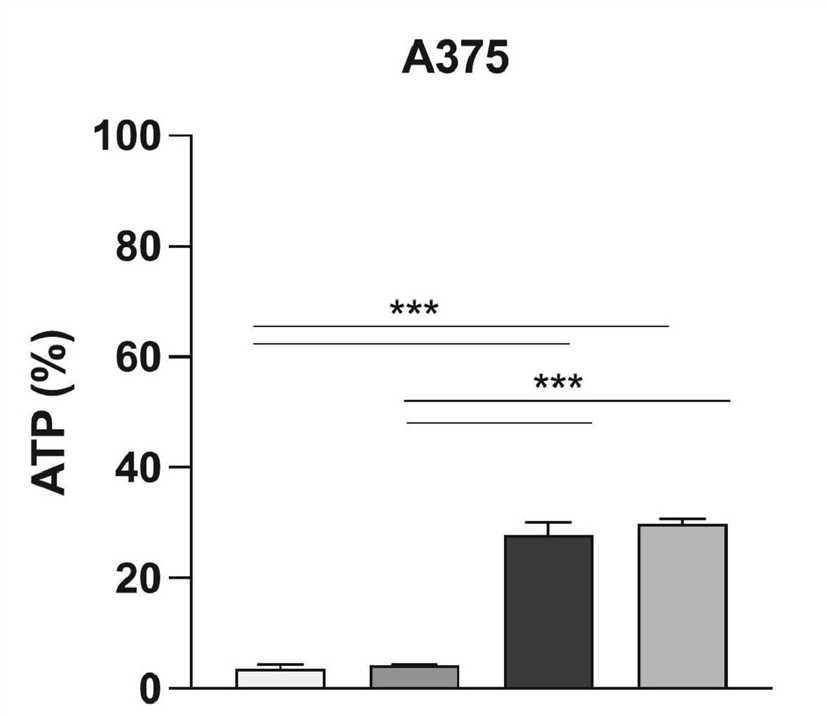 Fig.3 ATP levels are measured 72 h after infection.1
Fig.3 ATP levels are measured 72 h after infection.1
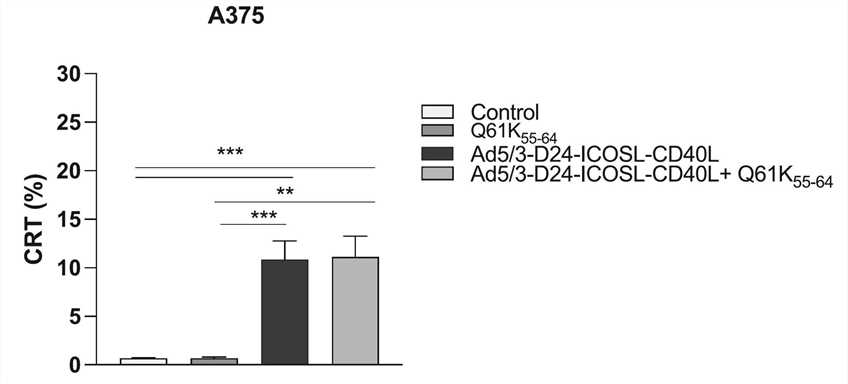 Fig.4 The expression of CRT in tumor cells is determined by flow cytometry.1
Fig.4 The expression of CRT in tumor cells is determined by flow cytometry.1
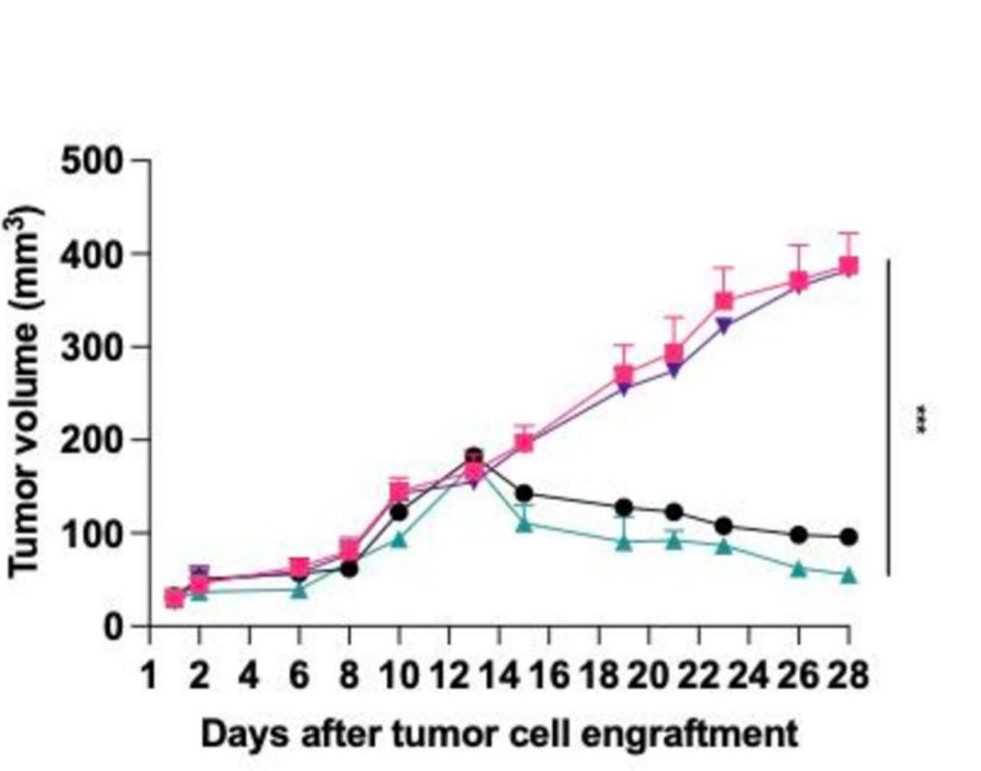 Fig.5 Tumor volume detection.1
Fig.5 Tumor volume detection.1
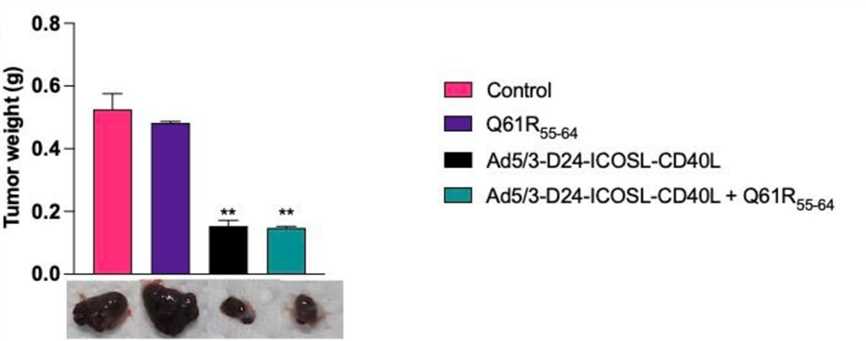 Fig.6 Tumor volume was measured at the end of the in vivo experiment.1
Fig.6 Tumor volume was measured at the end of the in vivo experiment.1