Adoptive T Cell & Oncolytic Virotherapy Combination Therapy Development Service
Accelerate Your Immuno-Oncology Research!
How Creative Biolabs Can Assist Your Project
In order to effectively improve the oncolytic effect of oncolytic virus (OV) and reduce side effects, the combination of oncolytic virus therapy and adoptive T cell therapy has become the preferred strategy. The safety and efficacy of combined therapy can be systematically evaluated through precise design of targeted oncolytic viruses that can specifically act on tumor cells, combined with comprehensive in vitro and in vivo experimental evaluation. This combined treatment mode can fully stimulate the anti-tumor immune response of the body, significantly improve the effect of cancer treatment, and provide a more potential treatment direction for overcoming cancer problems.
Creative Biolabs' OncoVirapy™ Platform provides clients with tailored solutions to enhance the efficacy of adoptive T-cell therapies. We deliver robust data packages and optimized oncolytic virus constructs to support your research program.
Workflow
| Required Starting Materials | Oncolytic Virus Design and Construction |
|---|---|
|
This includes, but is not limited to, providing the following information: Target tumor cell line information, including antigen expression profiles. Adoptive T-cell specifications (e.g., CAR design, TCR sequence). Requirements for in vivo models, if applicable (e.g., tumor model, immune status). |
We use specialized platforms to design and construct oncolytic viruses. This process involves genetic modification to enable the expression of specific cytokines and chemokines, which helps optimize their selective killing of tumor cells and immunomodulatory functions. |
| In Vitro Evaluation | In Vivo Model Development |
| The oncolytic virus is rigorously tested in vitro to assess its oncolytic activity, replication kinetics, and ability to enhance T-cell cytotoxicity. This includes assays such as viral titer determination, cell viability assays, and co-culture experiments with T cells | We develop appropriate in vivo models to evaluate the combination therapy. This may involve establishing syngeneic or xenograft tumor models and optimizing T-cell administration protocols |
| In Vivo Efficacy Studies | Immunological Assays |
| The efficacy of combined therapy is evaluated by establishing an in vivo model, monitoring tumor growth, assessing T cell infiltration into the tumor, and analyzing overall survival. | Flow cytometry, Elisa, immunohistochemistry, Western Blot and other immunological experiments are used to elucidate the mechanism of action and evaluate the effect of combined treatment on tumor microenvironment |
| Final Deliverables | Estimated Timeframe |
| Clients will receive detailed study reports with comprehensive data analysis, optimized oncolytic virus constructs, and in vitro and in vivo experimental results presented in a clear and concise manner. | The typical timeframe for this service ranges from 12 to 20 weeks, depending on the complexity of the oncolytic virus design, the specific requirements of the in vivo models, and the scope of the study. |
Why Choose Us?
Creative Biolabs is a leading service provider in oncolytic virotherapy and cancer immunotherapy. We offer:
- Expertise: Creative Biolabs possesses extensive knowledge in oncolytic virus design, construction, and in vivo evaluation. Our team has a deep understanding of virus-host interactions and the tumor microenvironment.
- Advanced Technology: Creative Biolabs utilizes cutting-edge technologies to develop and optimize oncolytic viruses for combination therapy. This includes proprietary platforms for virus engineering, vector design, and in vivo imaging.
- Customized Solutions: Creative Biolabs provides tailored solutions to meet the specific needs of each client's research program. We work closely with clients to design and execute studies that address their unique challenges and objectives.
- Comprehensive Services: Creative Biolabs offers a comprehensive range of services, from initial oncolytic virus design to final data analysis and reporting. We support both in vitro and in vivo studies.
Case Study
The application of combination therapy involving genetically modified oncolytic viruses and adoptive T-cell therapy in frequently used in vivo murine models and in vitro cancer cell line models, such as those for melanoma, has led to a substantial improvement in anti-tumor efficiency. Results from a multitude of published research works offer profound insights into its promising potential for treating various cancers, especially melanoma.
| Detection of Virus Replication Capacity | Cell Toxicity Assays | |
|---|---|---|
|
|
|
|
| Expression of Target Proteins | ||
|
|
||
| Cell Proliferation | ||
|
|
||
| Survival Curve | Analysis of immune cell infiltration | |
|
|
|
|
Customer Reviews:
-
"Enhanced T-cell activation: Using Creative Biolabs' Oncolytic Virotherapy service in our research has significantly improved T-cell activation and proliferation within the tumor microenvironment."
Dr. Chr***pherInstitution of Cancer Research
-
"Improved tumor regression: Creative Biolabs' oncolytic virus constructs demonstrated potent oncolytic activity and significantly improved tumor regression in our preclinical models."
Dr. Emily Br***wnBiotech Company
-
"Accelerated timeline: Creative Biolabs' expertise and efficient workflow helped us accelerate our research timeline and achieve our project milestones faster than anticipated."
Dr. Dav***dUniversity
[Experience the CB Advantage-Get a Quote Today]
Introduction
Oncolytic viruses (OVs) are a promising class of therapeutic agents that promote anti-tumor responses through selective tumor cell killing and the induction of systemic anti-tumor immunity. OVs can be integrated into tumor immunotherapies as they target multiple steps within the cancer-immunity cycle. Preclinical and clinical studies have confirmed that combining OVs with anti-cancer therapies can improve therapeutic responses.
Oncolytic Virotherapy Development for Combination Therapy with Adoptive T-Cell Therapy Service
Adoptive cellular transfer (ACT) therapy is a form of treatment that endeavors to combat cancer by enhancing the inherent capabilities of T lymphocytes. Oncolytic viruses (OVs) can assist in ACT by priming both the systemic immune system and the local tumor microenvironment (TME), thereby enabling better T cell infiltration and effector activity. Creative Biolabs has created a comprehensive immunotherapy platform for integrating oncolytic virotherapy with adoptive cellular transfer therapy, which is tailored to diverse disease types.
Tab.1 Preclinical studies combining oncolytic viruses with CAR-T cells.2,3
| Oncolytic Virus | CAR Antigen | CAR Endodomain | Tumor | |
|---|---|---|---|---|
| Adenovirus | Onc.Ad-EGFR bispecific antibody | Folate receptor alpha | 41BB | Pancreatic ductal carcinoma/colorectal carcinoma |
| Onc.Ad-TNFα/IL2 | Mesothelin (meso) | 41BB | Pancreatic ductal carcinoma | |
|
Onc.Ad- Rantes/lL15 |
Ganglioside GD2 | CD28, OX40 | Neuroblastoma | |
| CAdVEC-αPDL1 | HER2 | CD28 | Prostate, Squamous Cell Carcinoma | |
|
CAdVEC- IL12p70/αPDL1 |
Head and neck squamous cell carcinoma | |||
References
- Krabbe, Teresa, et al. "Adoptive T cell therapy is complemented by oncolytic virotherapy with fusogenic VSV-NDV in combination treatment of murine melanoma." Cancers 13.5 (2021): 1044. DOI: 10.3390/cancers13051044.
- Rosewell Shaw, Amanda, and Masataka Suzuki. "Oncolytic viruses partner with T-cell therapy for solid tumor treatment." Frontiers in immunology 9 (2018): 2103. DOI: 10.3389/fimmu.2018.02103.
- Distributed under Open Access license CC BY 4.0, Some of the pictures were edited and reformatted.

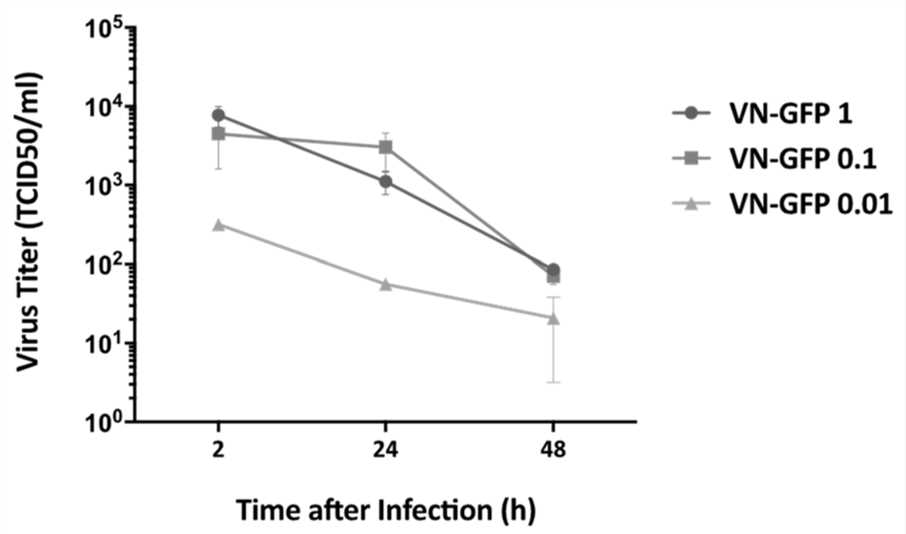 Fig.1 Viral titer determination.1,3
Fig.1 Viral titer determination.1,3
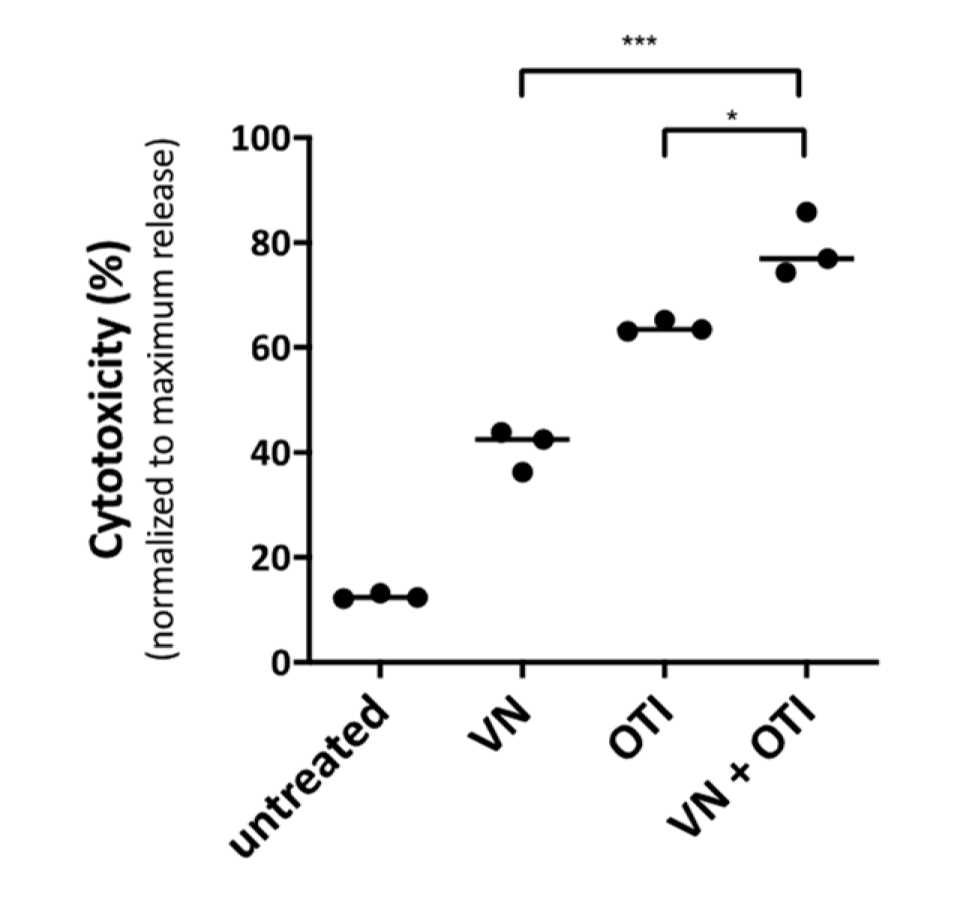 Fig.2 Cytotoxicity.1,3
Fig.2 Cytotoxicity.1,3
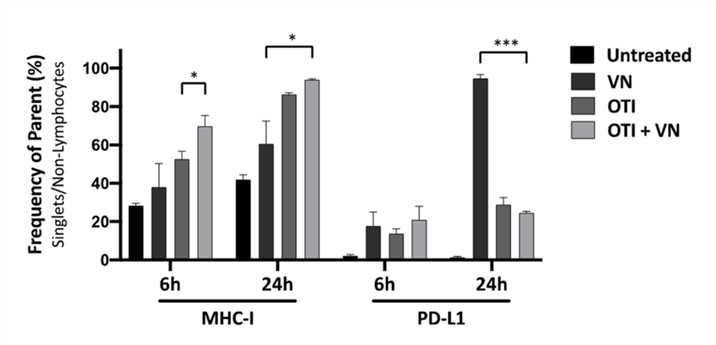 Fig.3 Flow cytometry is used to detect the expression of related indicators.1,3
Fig.3 Flow cytometry is used to detect the expression of related indicators.1,3
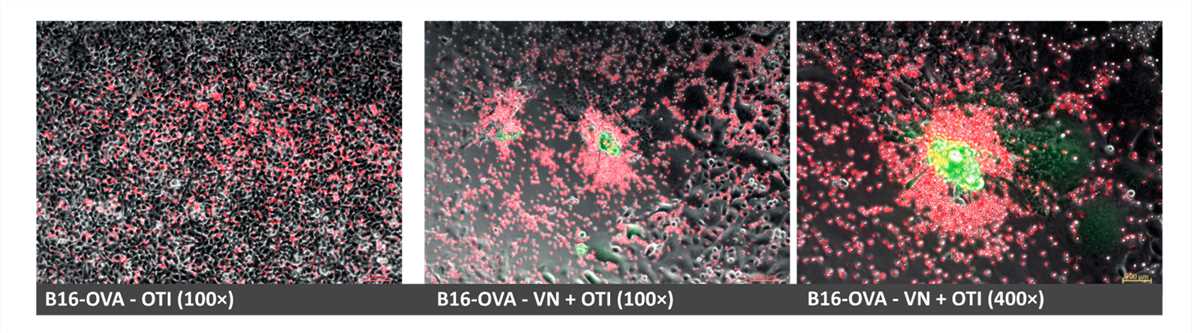 Fig.4 Cell proliferation is observed by fluorescence microscopy.1,3
Fig.4 Cell proliferation is observed by fluorescence microscopy.1,3
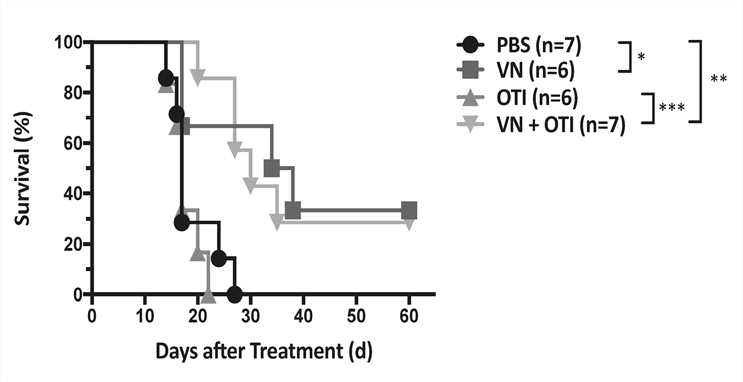 Fig.5 Survival of tumor-bearing mice.1,3
Fig.5 Survival of tumor-bearing mice.1,3
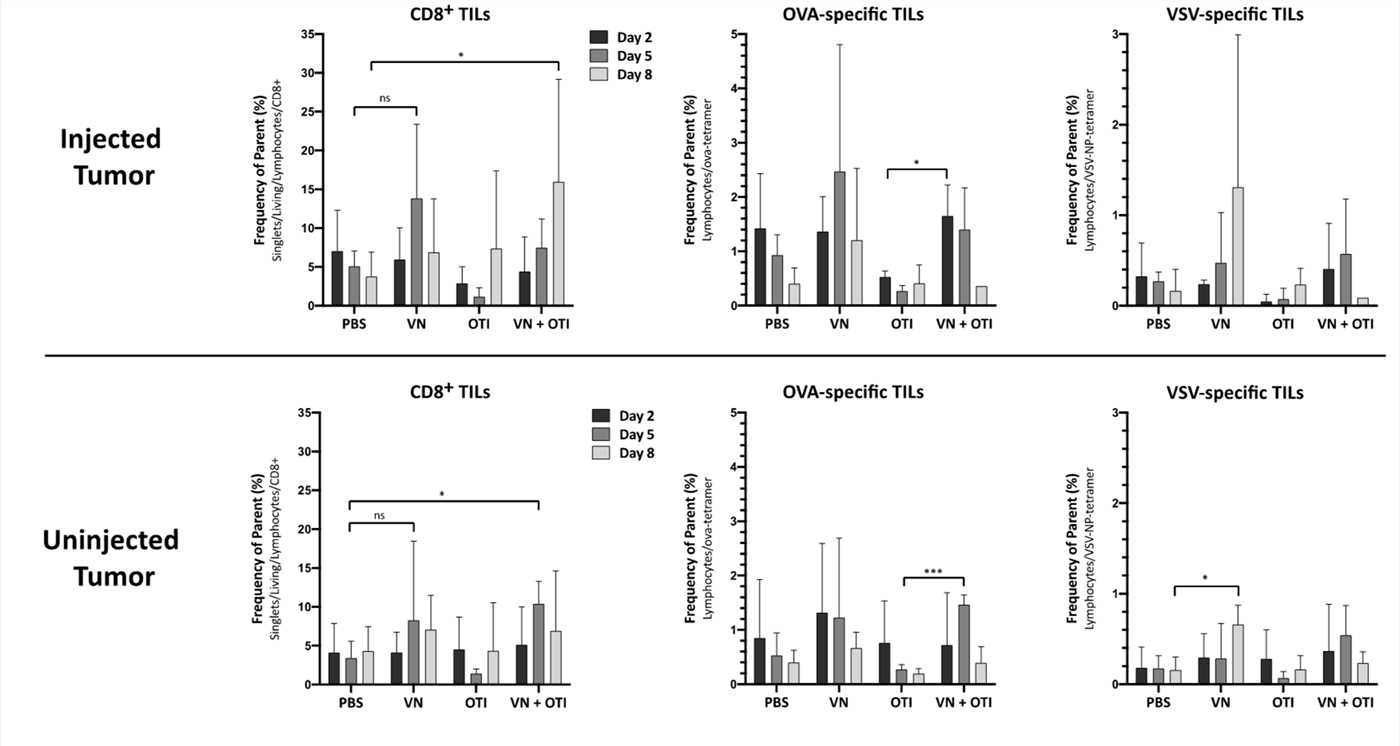 Fig.6 Tumor infiltration by immune cells.1,3
Fig.6 Tumor infiltration by immune cells.1,3