Melanoma Specific Oncolytic Virotherapy Development Service
Introduction
Melanoma is an aggressive and highly lethal disease, prone to metastasize to the lungs and even throughout the body. Currently, the optimal treatment modalities for advanced melanoma encompass targeted chemotherapy, oncolytic viruses (OVs) application, and immune checkpoint inhibition (ICI). In the context of primary melanoma treatment, OV therapy holds great promise. OV replication can induce remodeling of tumor cell lysis and microenvironment (TME). It also promotes the repolarization and recruitment of immune cells, thereby triggering secondary immune-mediated oncolysis. Creative Biolabs furnishes services for the development of oncolytic virotherapy for melanoma. Leveraging our well-established OncoVirapy™ platform, we integrate the entire therapy development process, spanning from oncolytic virus construction to in vitro and in vivo functional validation.
Introduction to Melanoma and The Structural Elements of The Skin
The skin is divided into three layers: the epidermis, the dermis, and the hypodermis.
- Epidermis: The epidermis typically consists of four layers: the stratum basale, the stratum spinosum, the stratum granulosum, and the stratum corneum.
- Dermis: Its cellular components include fibroblasts, macrophages, and adipocytes.
- Hypodermis: The hypodermis is the deepest layer, which is composed of the main branches of the skin circulatory system, sweat glands, and adipose tissues that produce vitamin D and triglycerides.
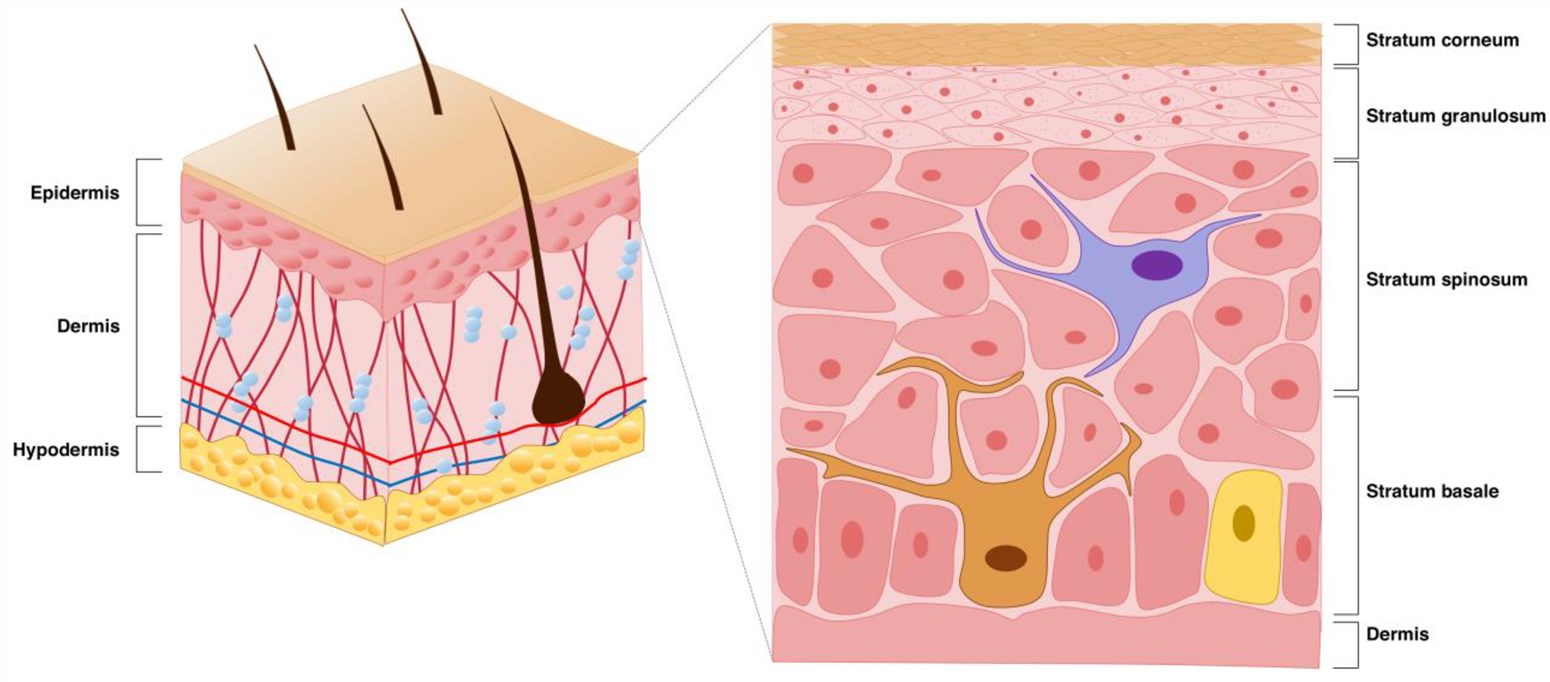 Fig.1 Schematic representation of the skin structure, where the brown cells on the right are melanocytes.1,3
Fig.1 Schematic representation of the skin structure, where the brown cells on the right are melanocytes.1,3
 Distributed under public domain, from Wiki, without modification
Distributed under public domain, from Wiki, without modification
Melanoma is induced by the transformation and unregulated proliferation of melanocytes in the basal layer of the skin. It can arise not only in the skin but also in the eyes, mucous membranes, and other cutaneous-related areas. Long-term ultraviolet exposure and a family history of the disease are associated risk factors.
Melanoma comprises four principal subtypes: superficial spreading melanoma, lentigo malignant melanoma, acral lentiginous melanoma, and nodular melanoma.
Tab.1 Staging of melanoma.
| Staging System | Specific Type/Stage | Key Characteristics |
|---|---|---|
| TNM Staging System | T (Tumor) | Describes the thickness of the tumor (in millimeters) and whether ulceration (breakage of the skin on the tumor surface) is present. |
| N (Lymph Nodes) | Indicates whether melanoma has spread to lymph nodes and the extent of spread. | |
| M (Metastasis) | Indicates whether melanoma has spread to other parts of the body (advanced or metastatic cancer). | |
| Numerical Staging System | Stage 0 (Melanoma in situ) | Cancer cells are only present in the outermost layer of the skin (epidermis) and have not spread to the dermis, with no risk of spread. |
| Stage 1 | Thickness ≤ 2 mm, no spread. | |
| Stage 1A | Thickness < 1 mm, no ulceration. | |
| Stage 1B | Thickness < 1 mm with ulceration; or thickness 1–2 mm, no ulceration. | |
| Stage 2 | Thicker than Stage 1, no spread. | |
| Stage 2A | Thickness 1–2 mm with ulceration; or thickness 2–4 mm, no ulceration. | |
| Stage 2B | Thickness 2–4 mm with ulceration; or thickness > 4 mm, no ulceration. | |
| Stage 2C | Thickness > 4 mm with ulceration. | |
| Stage 3 | Has spread to nearby lymphatic vessels or lymph nodes but not to other parts of the body (usually thick). Subdivided into Stages 3A, 3B, 3C, and 3D (based on the number of affected lymph nodes, visibility of cancer cells, and whether melanoma cells are found in nearby skin or lymphatic vessels). | |
| Stage 4 (Metastatic Melanoma) | Has spread to other organs (e.g., lungs, liver, bones, brain). |
Major Mutations and The Targeted Therapy in Melanoma Development
The main mutations that occur during melanoma development include BRAF mutations. Additionally, NRAS mutations, TERT promoter mutations, and the activation of the MAPK signaling pathway can be targeted for therapeutic intervention.
| Target | Mutation | Targeted Therapy |
|---|---|---|
| BRAF Mutations | A point mutation at codon 600 results in the substitution of glutamate for valine. Mutated BRAF impacts tumor survival and proliferation via downstream MAPK effector proteins and is likewise linked to augmented tissue invasion, cell migration, metastasis, and immune evasion. | BRAF inhibitors (BRAFis) such as vemurafenib and dabrafenib. |
| NARS Mutations | NRAS mutations are present in approximately 20% of wild-type BRAF melanomas. Results in defective GTPase activity and accumulation of RAS-GTP. Melanomas carrying NRAS mutations tend to be associated with more aggressive tumors and poor health. | siRNAs represent an alternative means for targeting NRAS-mutated melanomas. This strategy has been verified in pre-clinical models. However, owing to the instability of nucleic acids in the bloodstream, their delivery poses a substantial challenge. |
| Activation of MAPK pathway |
BRAF-mutated melanomas activate MAPK effector proteins, affecting tumor survival. Melanomas with NRAS mutations are dependent on CRAF signaling. |
Targeting downstream components of the MAPK signaling pathway with MEK inhibitors reduces drug resistance. |
| TERT promoter mutations | TERT mutations might be directly triggered by UV-induced damage. This creates binding motifs for ETS transcription factors, which enhance TERT transcriptional activity by a factor of two to four. The elevated transcriptional activity essentially leads to augmented telomerase production, thereby giving rise to immortalized, transformed melanocytes. |
Oncolytic virus therapy for melanoma
- T-VEC
Talimogene laherparepvec (T-VEC), a modified attenuated virus, is administered via intratumoral injection to stimulate the immune system in patients with postoperative recurrent melanoma. Fatigue is a common side effect, and in some cases, it may be associated with the risk of infection and autoimmune reactions. The combination of T-VEC and PD-1 antibodies demonstrates a remarkable effect in the treatment of malignant melanoma. Currently, this combination has also shown promising progress in the treatment of advanced sarcoma.
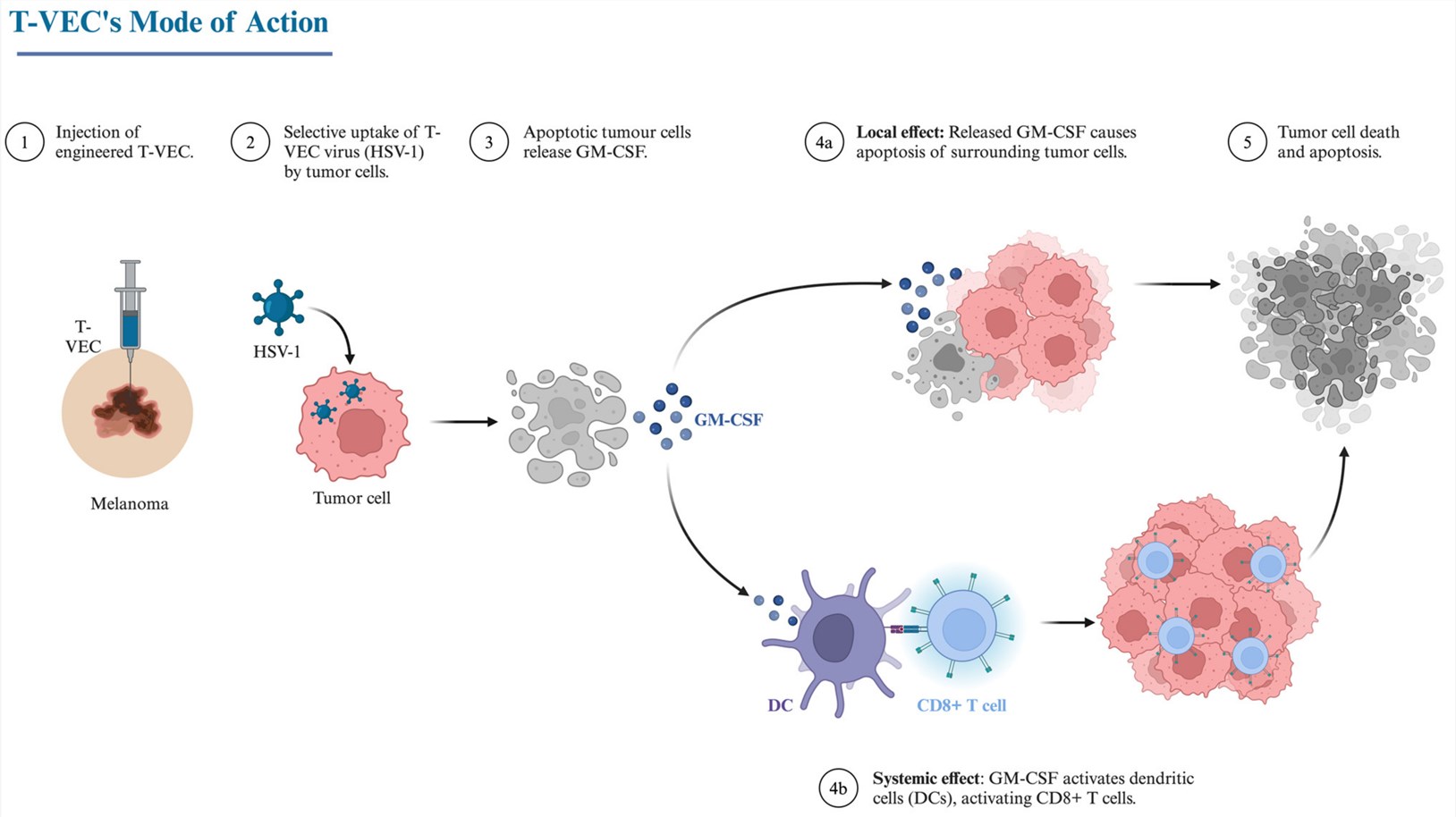 Fig.2 Mode of treatment of melanoma by T-VEC.2,3
Fig.2 Mode of treatment of melanoma by T-VEC.2,3
- OH2
OH2 is a genetically engineered oncolytic HSV-2. It has had the ICP34.5 and ICP47 genes removed to attenuate its virulence and enhance its oncolytic activity, and a GM-CSF expression cassette has been inserted to augment immune responses. Currently, in the ongoing phase Ⅰa/Ⅰb clinical trials, the safety and preliminary efficacy of OH2 are being evaluated. These trials have demonstrated good safety and durable anti-tumor efficacy in melanoma patients, particularly in those with progression despite anti-PD-1 therapy.
Workflow
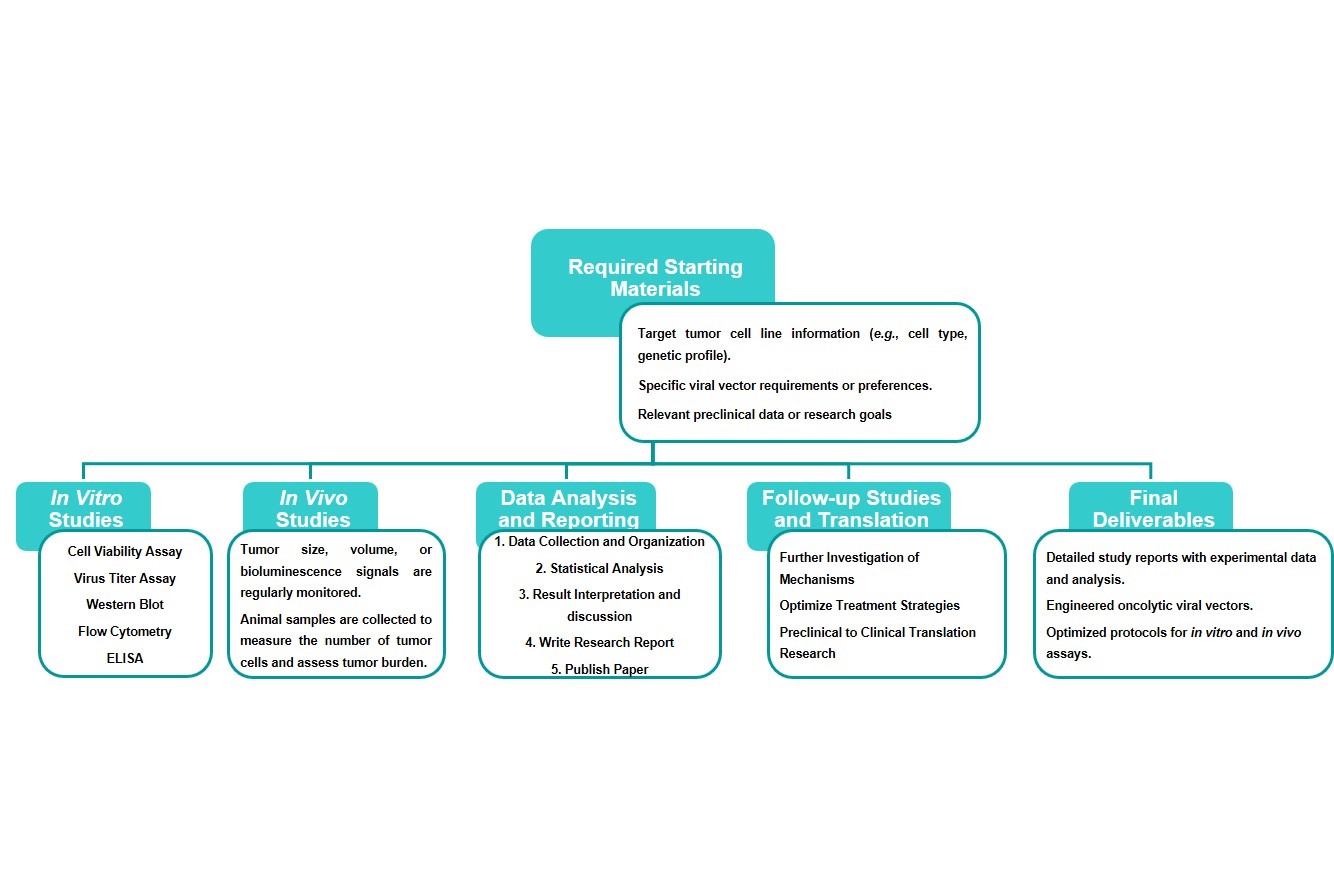
Estimated Timeframe:
Pre-requirement communication: 1-2 weeks
Design and construction of oncolytic viruses: 3-4 weeks
Mass production of oncolytic viruses: 2-3 weeks
Function and properties of oncolytic viruses in vivo and in vitro: 3-4 weeks
Results analysis and test report: 1-2 weeks
Product delivery and shipping: 2-3 weeks
Case Study
The utilization of genetically-modified oncolytic viruses in commonly employed in vivo murine models and in vitro melanoma cell line models has led to a remarkable enhancement in tumor-killing efficacy. Information collected from a multitude of scientific publications offers significant insights into its promising potential for melanoma treatment.
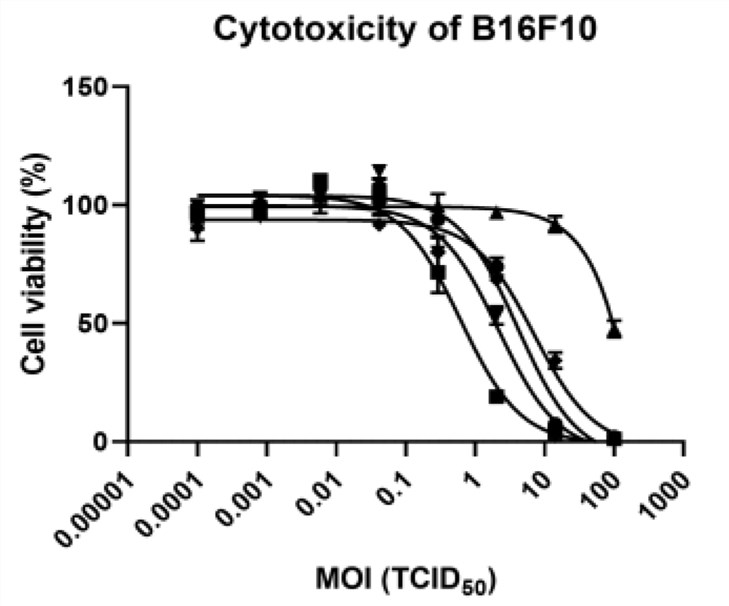 Fig.3 The cytotoxicity of oncolytic viruses in melanoma cells is determined by WST assay.4
Fig.3 The cytotoxicity of oncolytic viruses in melanoma cells is determined by WST assay.4
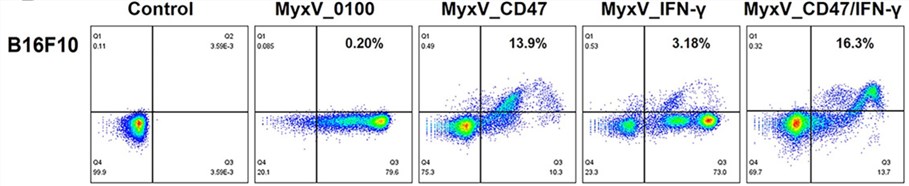 Fig.4 The expression of oncolytic protein-armed genes is detected by flow cytometry.4
Fig.4 The expression of oncolytic protein-armed genes is detected by flow cytometry.4
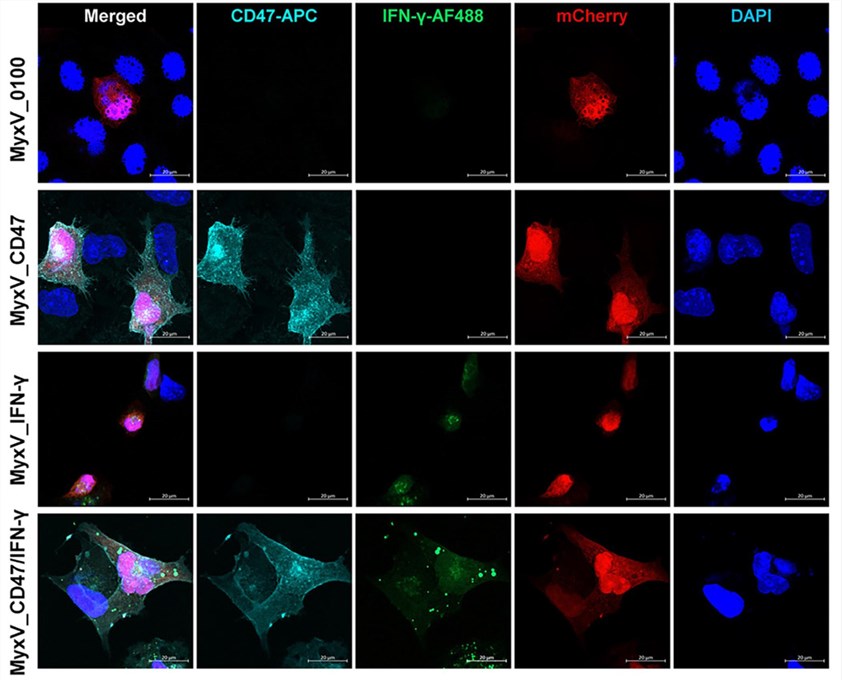 Fig.5 Immunofluorescence is used to observe the expression of target proteins.4
Fig.5 Immunofluorescence is used to observe the expression of target proteins.4
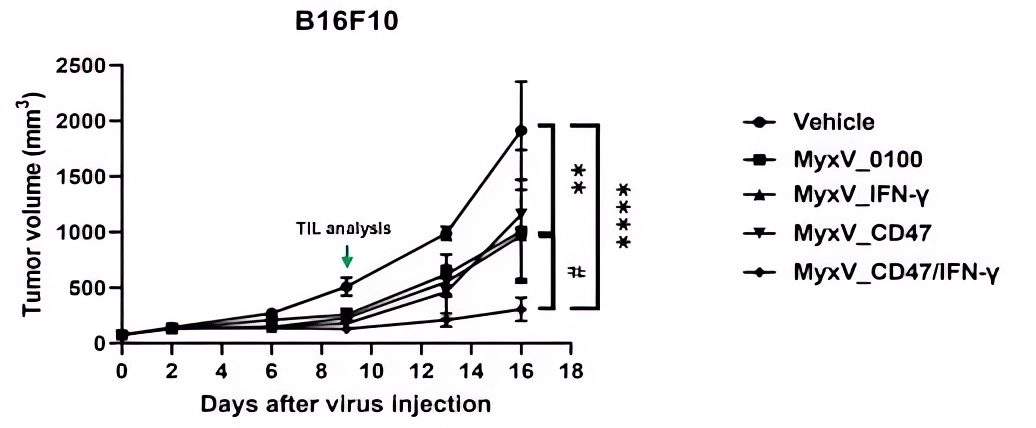 Fig.6 The Oncolytic virus effectively reduced the volume of melanoma.4
Fig.6 The Oncolytic virus effectively reduced the volume of melanoma.4
References
- Brito, Sofia, Moonki Baek, and Bum-Ho Bin. "Skin structure, physiology, and pathology in topical and transdermal drug delivery." Pharmaceutics 16.11 (2024): 1403. DOI: 10.3390/pharmaceutics16111403
- Kulbay, Merve, et al. "Oncolytic Viruses and Immunotherapy for the Treatment of Uveal Melanoma and Retinoblastoma: The Current Landscape and Novel Advances." Biomedicines 13.1 (2025): 108. DOI: 10.3390/biomedicines13010108
- Distributed under Open Access license CC BY 4.0, without modification.
- Woo, Jong Kyu, et al. "Dual-Armed oncolytic myxoma virus encoding IFN-γ and CD47 promotes Lymphocyte infiltration and tumor suppression of syngeneic murine melanoma." Cancers 15.19 (2023): 4703. DOI: 10.3390/cancers15194703. Distributed under Open Access license CC BY 4.0, reformat the picture.
