Lung Cancer Specific Oncolytic Virotherapy Development Service
Introduction
Lung cancer is one of the malignant tumors with the highest occurrence and fatality rates. Traditional treatment methods include radiation therapy, chemotherapy, surgical removal, and other approaches. Oncolytic viral therapy (OV), a type of cancer immunotherapy, shows broad anti-tumor effects. The idea of using oncolytic viruses to treat cancer has been verified in many recent clinical trials. The findings indicate that patients undergoing oncolytic adenovirus treatment alone or in combination with chemotherapy have much higher objective response rates than those who only receive chemotherapy. This suggests that OV holds broad research prospects in the realm of lung cancer.
With Creative Biolabs' accurate gene regulation oncolytic virus development platform OncoVirapy™ and experienced scientists' deep understanding of lung cancer-related gene mutations, we can create OV therapies targeting specific gene mutations, and provide customers with gene-modified oncolytic virus construction and in vitro efficacy validation services.
Factors Predisposing to Lung Cancer
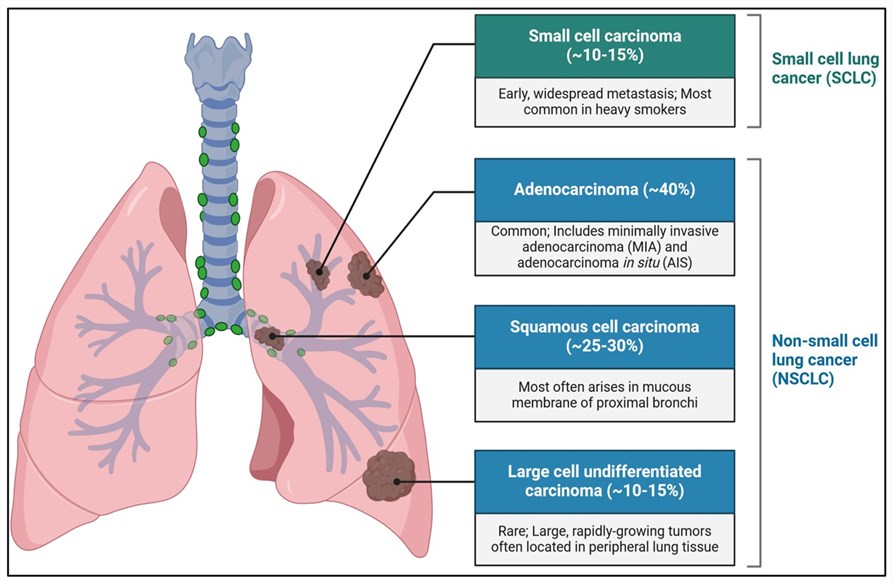 Fig.1 The main types of lung cancer are NSCLC and SCLC.1,3
Fig.1 The main types of lung cancer are NSCLC and SCLC.1,3
Tab.1 Classification of lung cancer as well as predisposing factors, common symptoms, and treatments.
| Non-small-cell Lung Cancers (NSCLC) | Small-cell Lung Cancer (SCLC) | ||
|---|---|---|---|
| Approximately 85% of lung cancers are NSCLC, which generally grows at a slower rate compared to SCLC. However, 40% of NSCLC patients are diagnosed when the tumors have metastasized beyond the chest. The most prevalent histological subtypes of NSCLC are squamous cell carcinoma, adenocarcinoma, and large-cell carcinoma. | SCLC, sometimes called oat cell carcinoma, constitutes approximately 15% of all lung cancer incidences. Small-cell lung cancer is extremely aggressive and metastasizes rapidly. By the time of diagnosis, the majority of patients present with cancer that has metastasized to other body sites. | ||
| Lung cancer-related gene mutation |
Growth-stimulating genes: KRAS, MYC Genes related to growth factor receptor signaling pathway: EGFR, HER Tumor suppressor genes: TP53, APC Other related molecular marker genes: EML-4-ALK, ROS-1, BRAF, PIK3CA |
||
| Causes of lung cancer | Smoking represents the primary etiological factor of lung cancer, accounting for approximately 85% of all lung cancer incidences. Other possible risk factors include outdoor air contamination, exposure to tobacco smoke from active smoking and second-hand smoke, and breathing in cancer-causing substances like asbestos, sulfur mustard, or emissions from coke ovens. | ||
| Common symptoms | Chronic cough, Hemoptysis, Anorexia, Weight reduction, Fatigue, Chest pain, Malaise, Dysphagia, Horner syndrome, Arrhythmia | ||
| Tests and treatment options | Chest radiography can detect the majority of lung cancers. Nevertheless, more imaging techniques and tissue samples need to be taken to verify the diagnosis. In treating lung cancer, surgical removal, chemo treatment, precision medicines, immune-based therapies, and radiation treatment are all frequently used. | ||
Oncolytic Virus Therapy for Lung Cancer
To date, numerous OVs and engineered viral vectors, such as adenovirus, herpes simplex virus, vaccinia virus, coxsackievirus, reovirus, poliovirus, and measles virus, have entered early-stage clinical trials. Many of these have also demonstrated notable efficacy in the treatment of lung cancer.
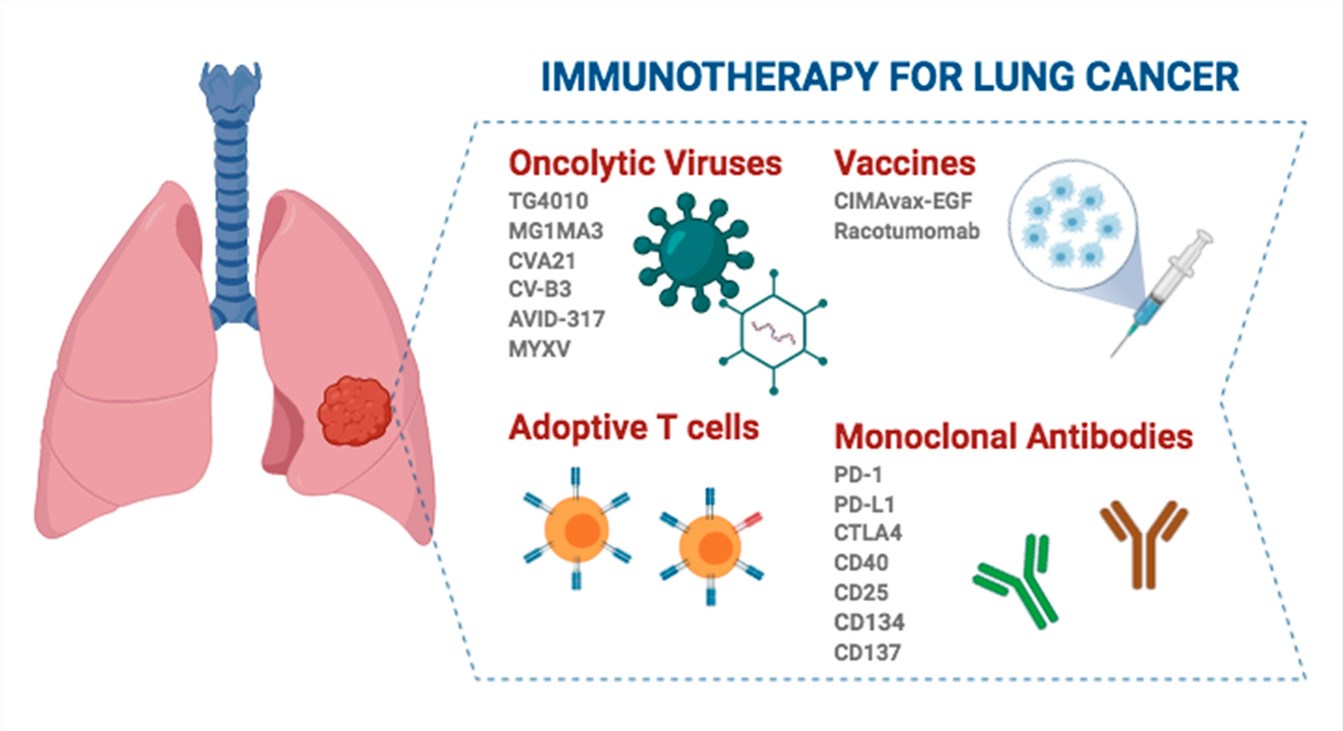 Fig.2 Different strategies for the treatment of lung cancer and the clinical application of immunotherapy drugs.2,3
Fig.2 Different strategies for the treatment of lung cancer and the clinical application of immunotherapy drugs.2,3
- MVA-MUC1-IL2: It is a modified Ankara vaccinia virus, genetically engineered to express MUC-1 and IL-2. When combined with CT, it improves progression-free survival and increases durable response and long-term survival in patients with advanced NSCLC. It has a synergistic effect when combined with an anti-PD-1/PD-L1 immune checkpoint inhibitor.
- MG1 Maraba/MAGE-A3 (MG1MA3) and the MAGE-A3 (Ad-MAGEA3) adenovirus: MG1 Maraba expressing MAGE-A3 (MG1-MAGEA3) and the MAGE-A3 (Ad-MAGEA3) adenovirus vaccine in combination with Pembrolizumab are being used for the treatment of patients with metastatic NSCLC in a Phase 1/2 trial. They have achieved the efficacy of T cell-mediated tumor clearance and prevention of recurrence in the body, thus avoiding host-related antiviral responses.
- CVA21: The oncolytic virus, Coxsackie virus A21 (CVA21), which naturally targets ICAM-1, is well-tolerated in combination with pembrolizumab and seemed to augment the number of PD-L1+ tumor cells in the context of Phase Ⅰb clinical trials.
- CV-B3: Coxsackievirus B3 shows potent oncolytic activity against KRAS-mutant lung adenocarcinoma. CVB3 replication depends on the ERK1/2 pathway. It can notably reduce the viability of KRAS-mutant NSCLC cell lines.
- AVID-317: AVID 317 employs short lamin-derived peptides to substitute the RGD loop in the Ad-C5 penton base, allowing the virus to be internalized into cells via α6 integrins and other integrins that interact with lamins. It has been demonstrated to be efficacious in 70% of human NSCLC-derived cell lines tested and has improved median survival in mouse models.
Workflow
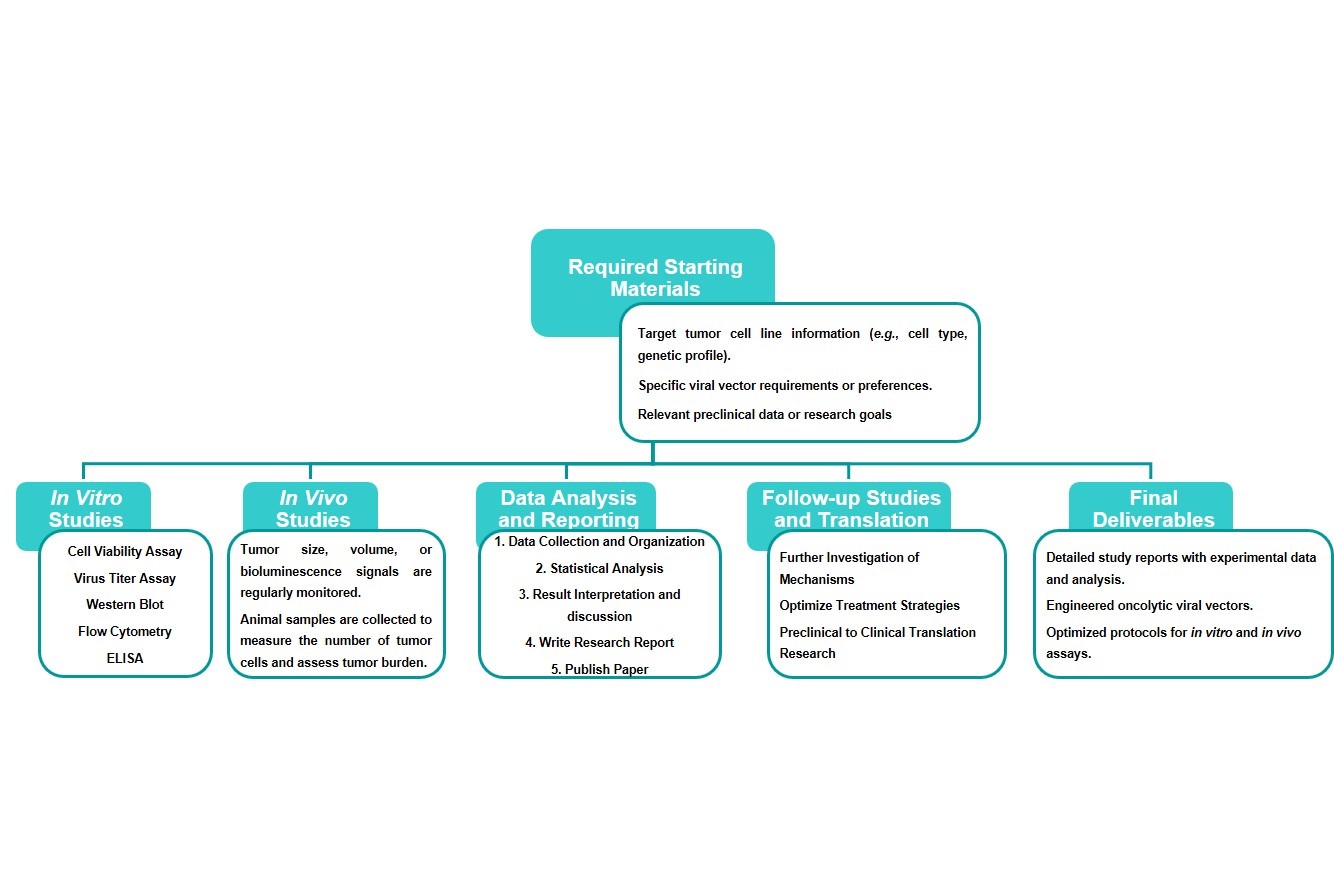
Estimated Timeframe:
Pre-requirement communication: 1-2 weeks
Design and construction of oncolytic viruses: 3-4 weeks
Mass production of oncolytic viruses: 2-3 weeks
Function and properties of oncolytic viruses in vivo and in vitro: 3-4 weeks
Results analysis and test report: 1-2 weeks
Product delivery and shipping: 2-3 weeks
Case Study
The use of oncolytic viruses in vivo murine models and in vitro cancer cell line models of lung cancer has resulted in a notable enhancement of tumor-killing rates. Evidence collected from multiple published scientific reports presents valuable outlooks on its promising future in treating lung cancers.
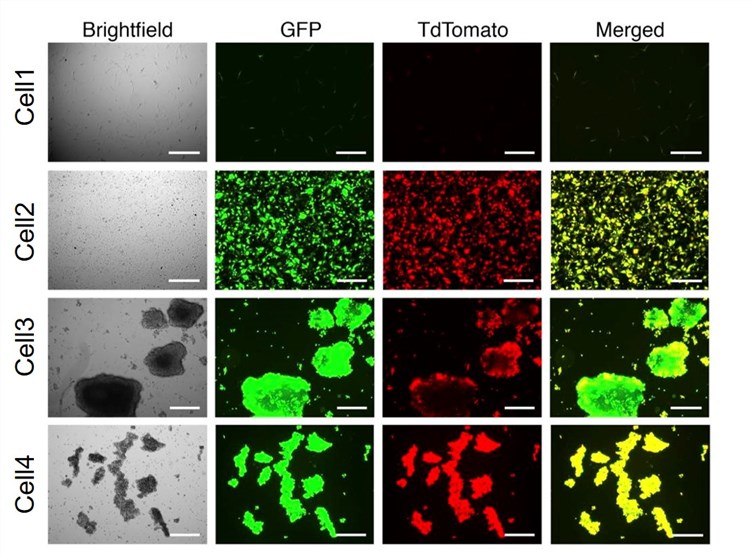 Fig.3 Oncolytic virus shows good early and late expression in different lung cancer cell lines.4
Fig.3 Oncolytic virus shows good early and late expression in different lung cancer cell lines.4
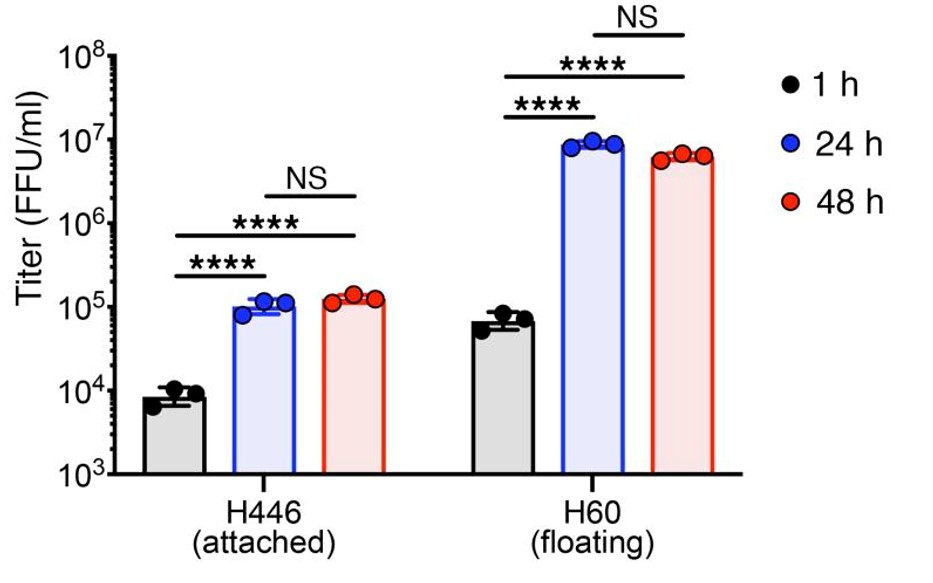 Fig.4 The replication ability of oncolytic virus in lung cancer cell lines increased with time.4
Fig.4 The replication ability of oncolytic virus in lung cancer cell lines increased with time.4
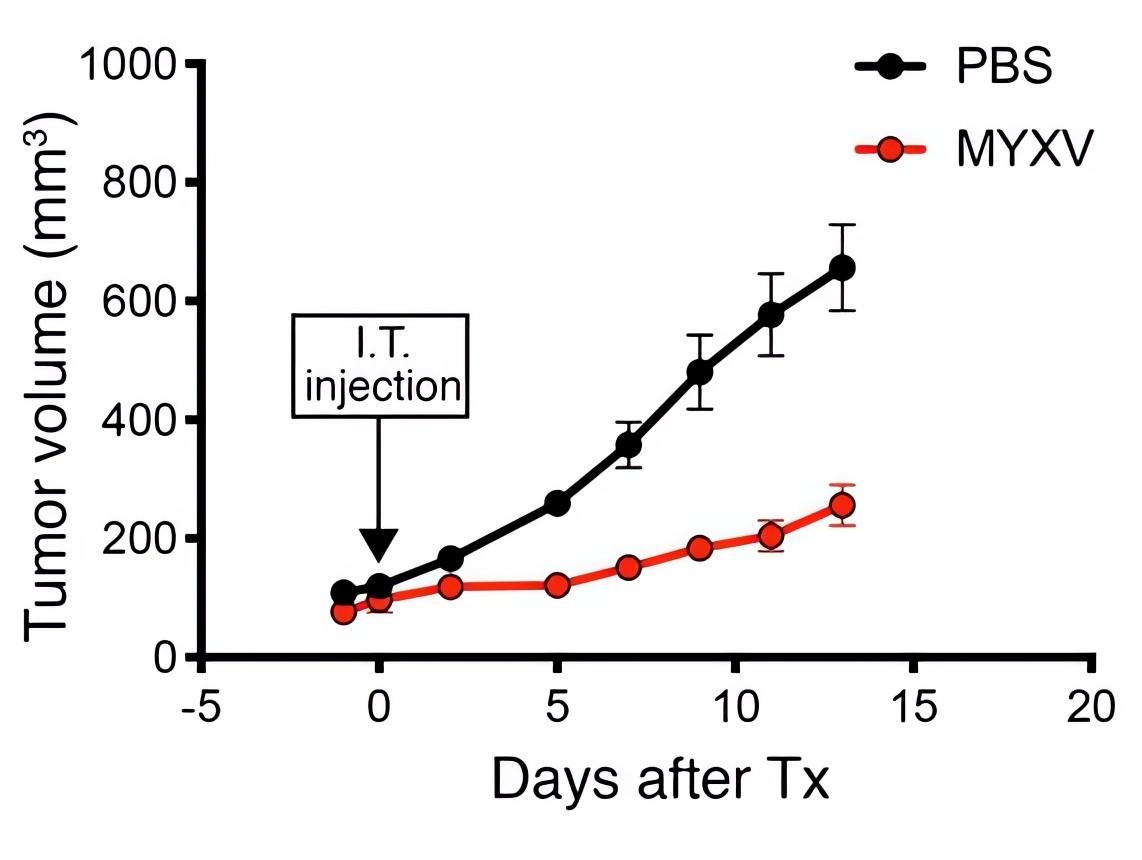
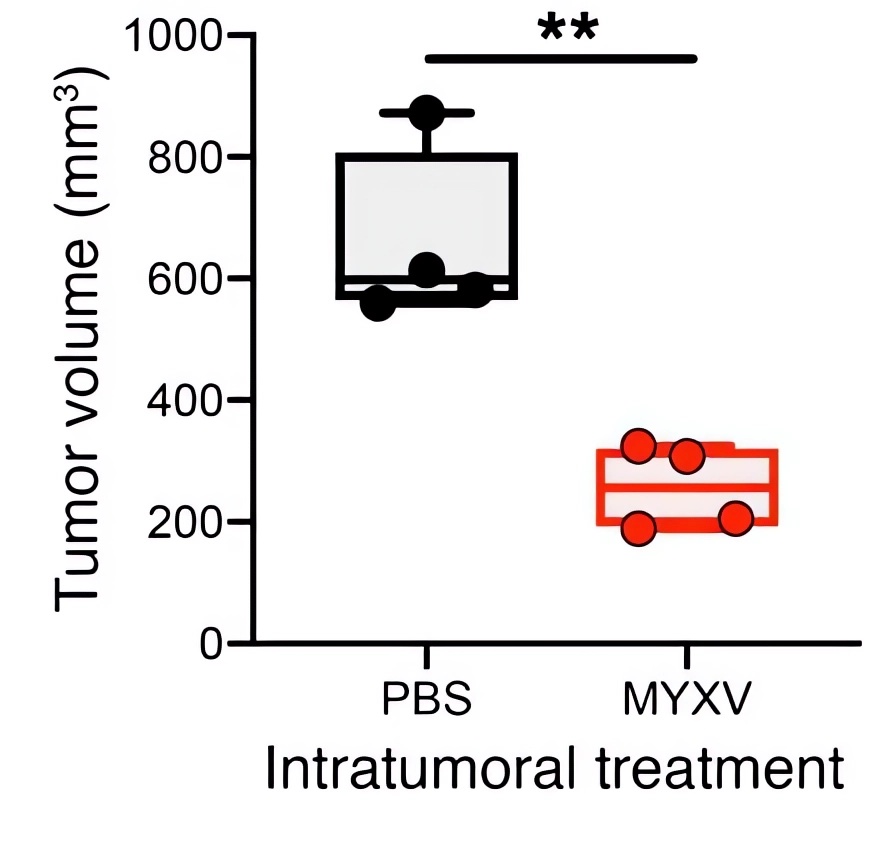
Fig.5 Oncolytic virotherapy significantly reduces lung tumor volume.4
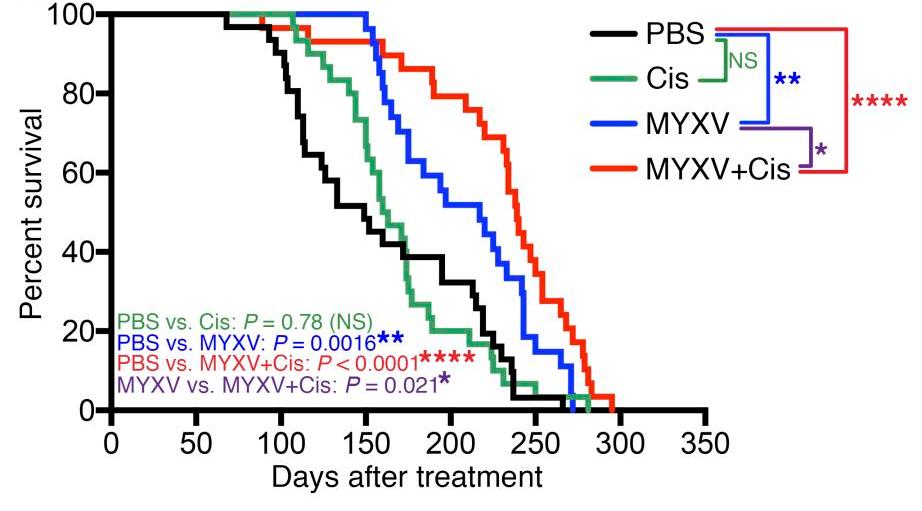 Fig.6 Oncolytic virotherapy prolongs survival in mice with cancer.4
Fig.6 Oncolytic virotherapy prolongs survival in mice with cancer.4
Creative Biolabs can provide high-quality and specialized oncolytic virus construction services and functional verification services tailored to customer requirements. These offerings can meet the needs for detecting in vivo and in vitro effects across a wide variety of tumor models. Should you have such requirements, feel free to reach out to us, we are pleased to provide our services.
References
- Garg, Pankaj, et al. "Advances in non-small cell lung cancer: current insights and future directions." Journal of clinical medicine 13.14 (2024): 4189. DOI: 10.3390/jcm13144189
- Boyero, Laura, et al. "Primary and acquired resistance to immunotherapy in lung cancer: unveiling the mechanisms underlying of immune checkpoint blockade therapy." Cancers 12.12 (2020): 3729. DOI: 10.3390/cancers12123729
- Distributed under Open Access license CC BY 4.0, without modification.
- Kellish, Patrick, et al. "Oncolytic virotherapy for small-cell lung cancer induces immune infiltration and prolongs survival." The Journal of clinical investigation 129.6 (2019): 2279-2292. DOI: 10.1172/JCI121323. Distributed under Open Access license CC BY 4.0, reformat the picture.
