Gynecologic Malignancy Specific Oncolytic Virotherapy Development Service
Introduction
Around the world, the number of women developing gynecological cancers such as ovarian, cervical, and endometrial cancer is steadily climbing, posing a serious threat to women's well-being. Ovarian cancer is particularly worrying because it often spreads to other parts of the body and can become resistant to drugs, making it extremely difficult to treat. At the same time, the rates of cervical and endometrial cancer are also increasing, and these cancers are showing up in younger women, which can have a big impact on their ability to have children and their overall quality of life.
At present, the primary treatment for these cancers involves a mix of surgical operations, radiotherapy, chemo treatments, and other therapeutic methods. Nevertheless, two major hurdles exist. For one, some cancers build up resistance to these therapies, gradually reducing their effectiveness. Moreover, early detection of these cancers is frequently difficult. When cancers aren't caught early, even with a combination of treatments, the results may fall short of what's hoped for.
Oncolytic viruses (OVs) are also getting a lot of attention. In the beginning, scientists used wild-type first-generation OVs. Thanks to advances in biotechnology, the field has now moved on to more sophisticated second and third-generation engineered OVs, which are designed to be even more effective against these gynecological cancers. Leveraging its professional oncolytic virus construction platform OncoVirapy™, Creative Biolabs is dedicated to the development of specialized and distinctive oncolytic virus-based treatment regimens. The company offers comprehensive oncolytic virus construction and validation services tailored for the treatment of gynecological malignancies.
The Common Types and Characteristics of Gynecologic Malignancies
| Types of Cancer | Main manifestations |
|---|---|
| Cervical cancer | The vast majority of cervical cancers are due to human papillomavirus (HPV) infection. About 80%-85% are squamous-cell carcinomas, originating from the flat, squamous cells of the cervical wall. |
| Uterine cancer | About 75%-80% of endometrial cancers are adenocarcinomas, arising from glandular cells. Uterine sarcomas are more aggressive than other endometrial tumors. |
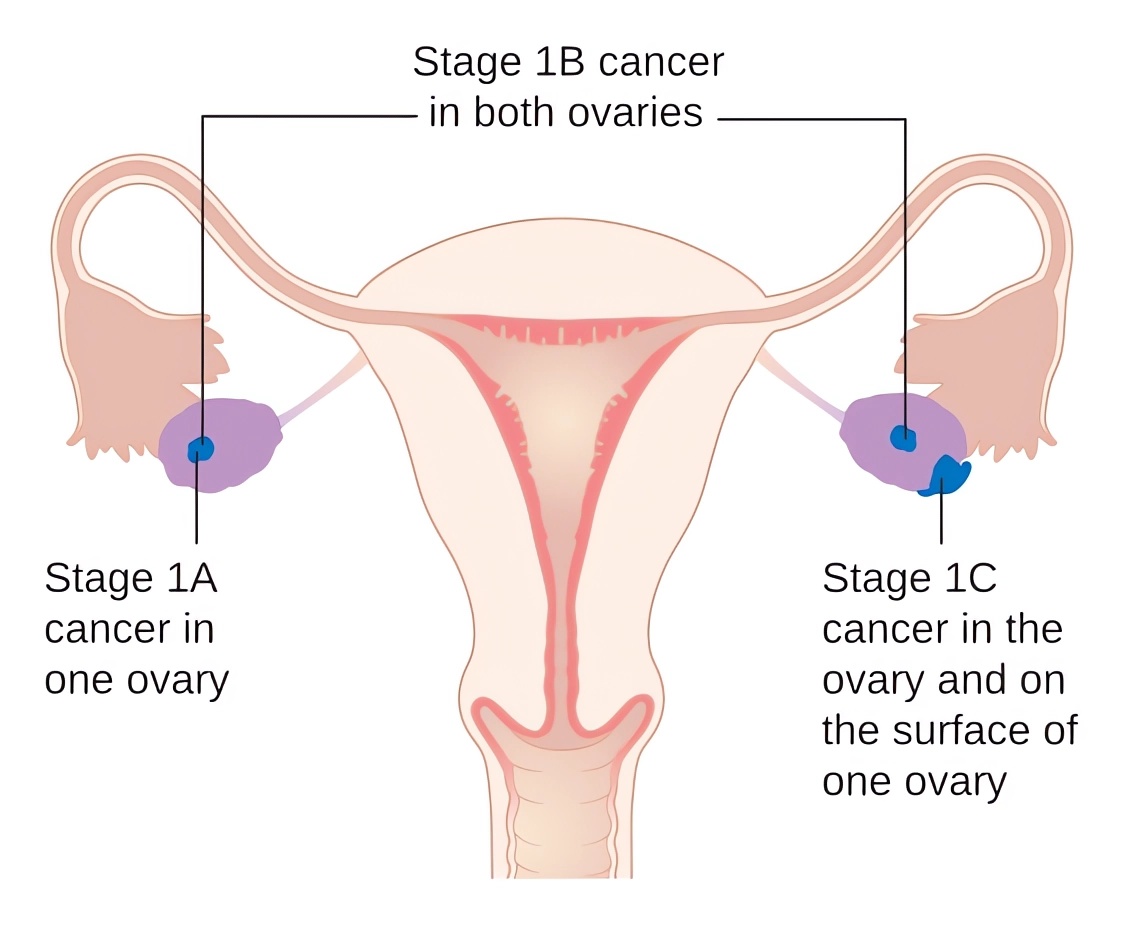
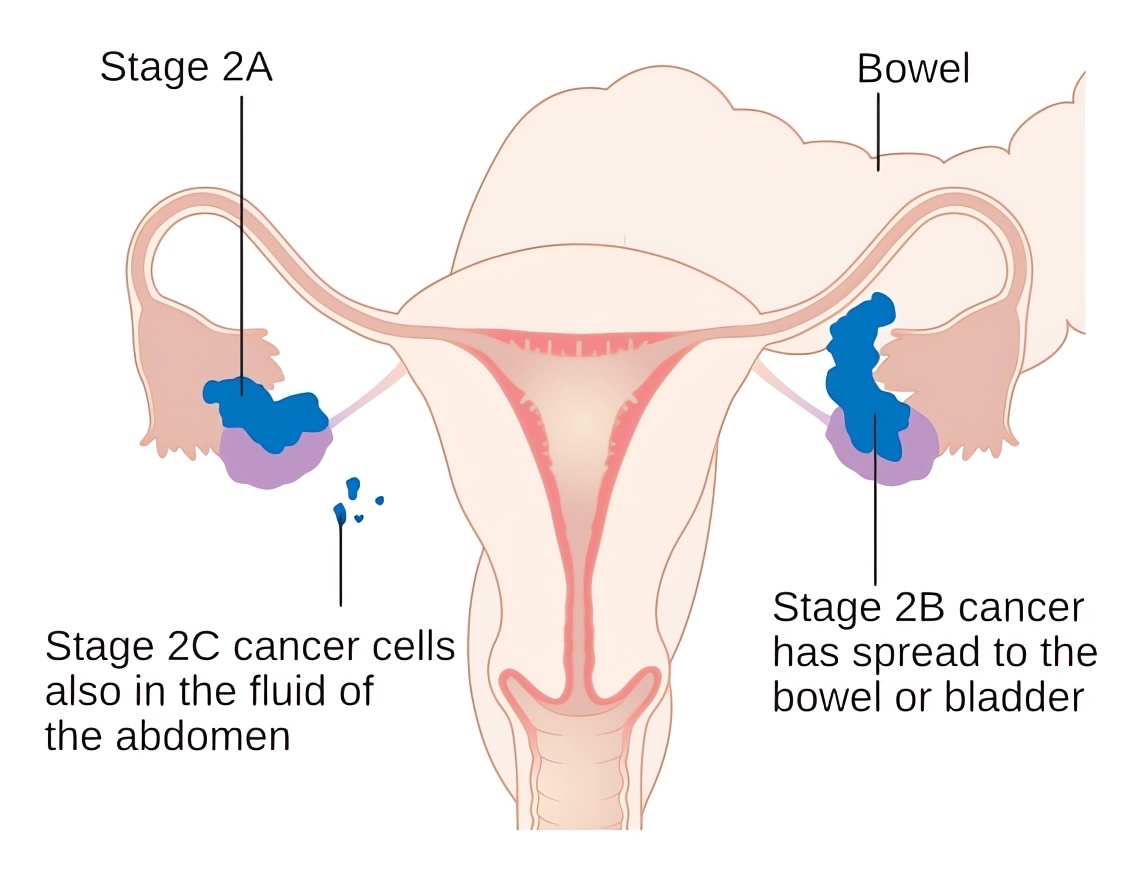
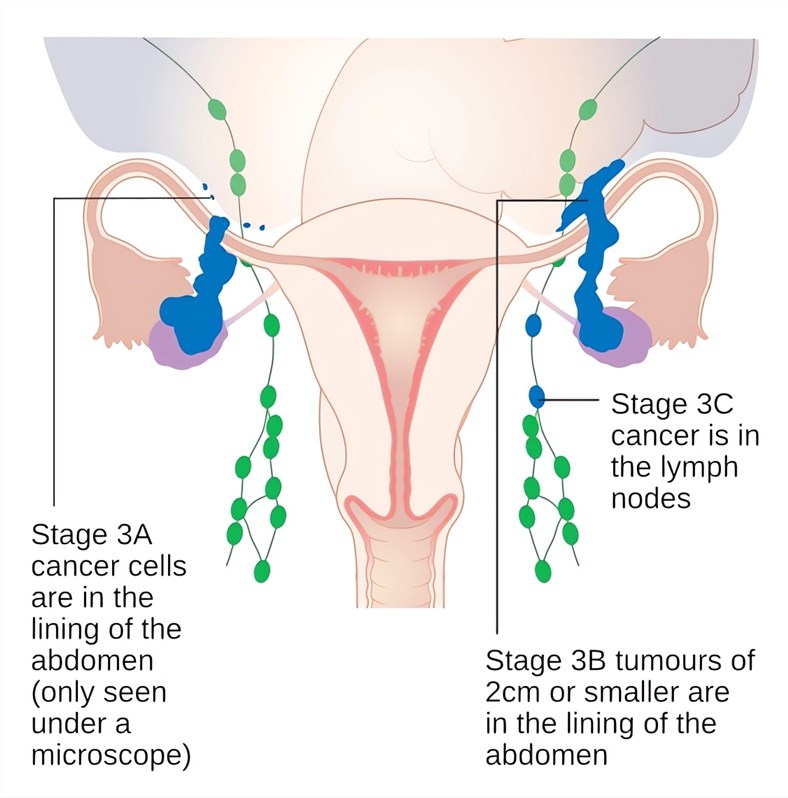
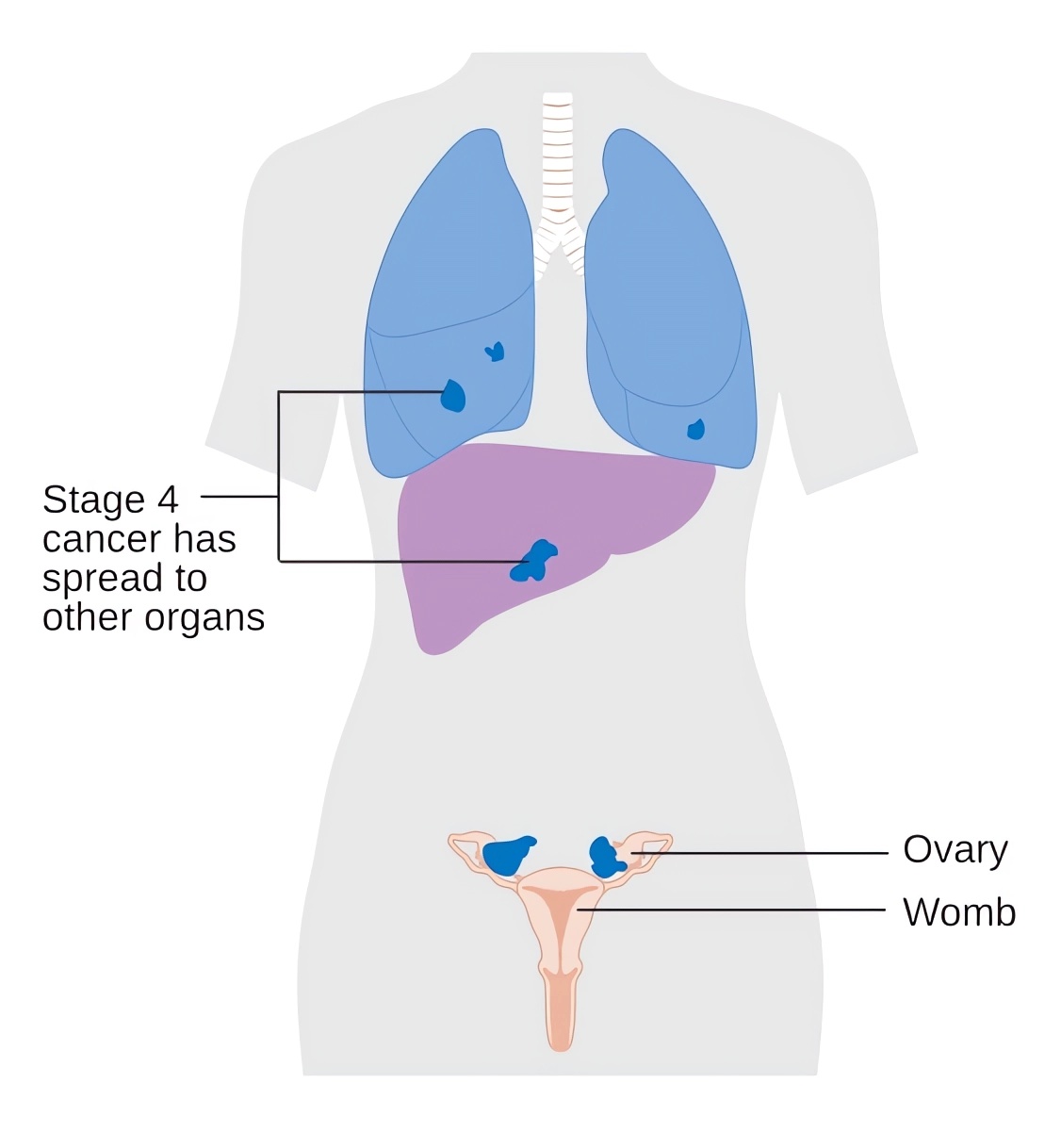
| Ovarian cancer | Approximately 15% of ovarian cancer cases are due to BRCA1/BRCA2 mutations. Ovarian cancer enlarges the affected ovary. Germ cell tumors can produce estrogen, causing endometrial and breast hyperplasia, or androgens, leading to virilization symptoms. |
 Distributed under CC BY-SA 4.0, from Wiki, without modification.
Distributed under CC BY-SA 4.0, from Wiki, without modification.
 Distributed under CC BY-SA 4.0, from Wiki, without modification.
Distributed under CC BY-SA 4.0, from Wiki, without modification.
 Distributed under CC BY-SA 4.0, from Wiki, without modification.
Distributed under CC BY-SA 4.0, from Wiki, without modification.
 Distributed under CC BY-SA 4.0, from Wiki, without modification.
Distributed under CC BY-SA 4.0, from Wiki, without modification.
Oncolytic Virus Therapy for Gynecologic Malignancies
- Oncolytic virus therapy for uterine cancer: H101 is a recombinant human type 5 oncolytic adenovirus in which the entire E1B-55 kDa gene and a 19 kDa fragment of the E3 region have been deleted. Intratumoral injection of H101 in combination with radiotherapy demonstrates favorable tolerability and oncolytic efficacy.
- Oncolytic virus therapy for cervical cancer: Hu et al. employed a gene-editing system integrated with recombinant oHSV-1 to precisely target and disrupt the integrated HPV16 gene within cervical cancer cells. This approach specifically and efficiently downregulated the HPV16 oncogene, consequently reducing cell proliferation and promoting apoptosis1.
 Distributed under CC BY 3.0, from Wiki, without modification.
Distributed under CC BY 3.0, from Wiki, without modification.
Researchers have developed several oncolytic adenovirus viruses that show great promise in treating cervical cancer. These include Ad5-Delta24-RGD, which contains αvβ3 and αvβ5 integrin-binding RGD motifs, the E1 A and E1 B double-mutant Ad-DeltaE1Bmt7, Ad-URR/E1ADelta24, driven by the upstream regulatory promoter region (URR) of HPV-16; and Ad-KFH, which is transcriptionally controlled by the SCCA 2 promoter. When delivered intratumorally or intravenously in a cervical cancer xenograft mouse model, these viruses can effectively inhibit tumor growth.
-
Oncolytic virus therapy for ovarian cancer:
RV effectively shrank intraperitoneal ovarian cancer tumors, inhibited ascites, and increased treated animals' survival. MeV-NIS can express NIS genes in infected cells and map proliferation via radioiodine imaging. In ovarian cancer mouse models, MeV-CEA achieved 80% tumor regression when injected subcutaneously and improved survival with intraperitoneal use. Oncolytic MeV retargeted to FRα enhanced tumor selectivity. Recombinant (TK) VV with lucZ and lacZ in the TK region showed efficient tumor-related activity and longer animal survival. In a mouse ovarian cancer model, vvDD incorporated with the CD suicide gene selectively targeted tumor cells.
Workflow
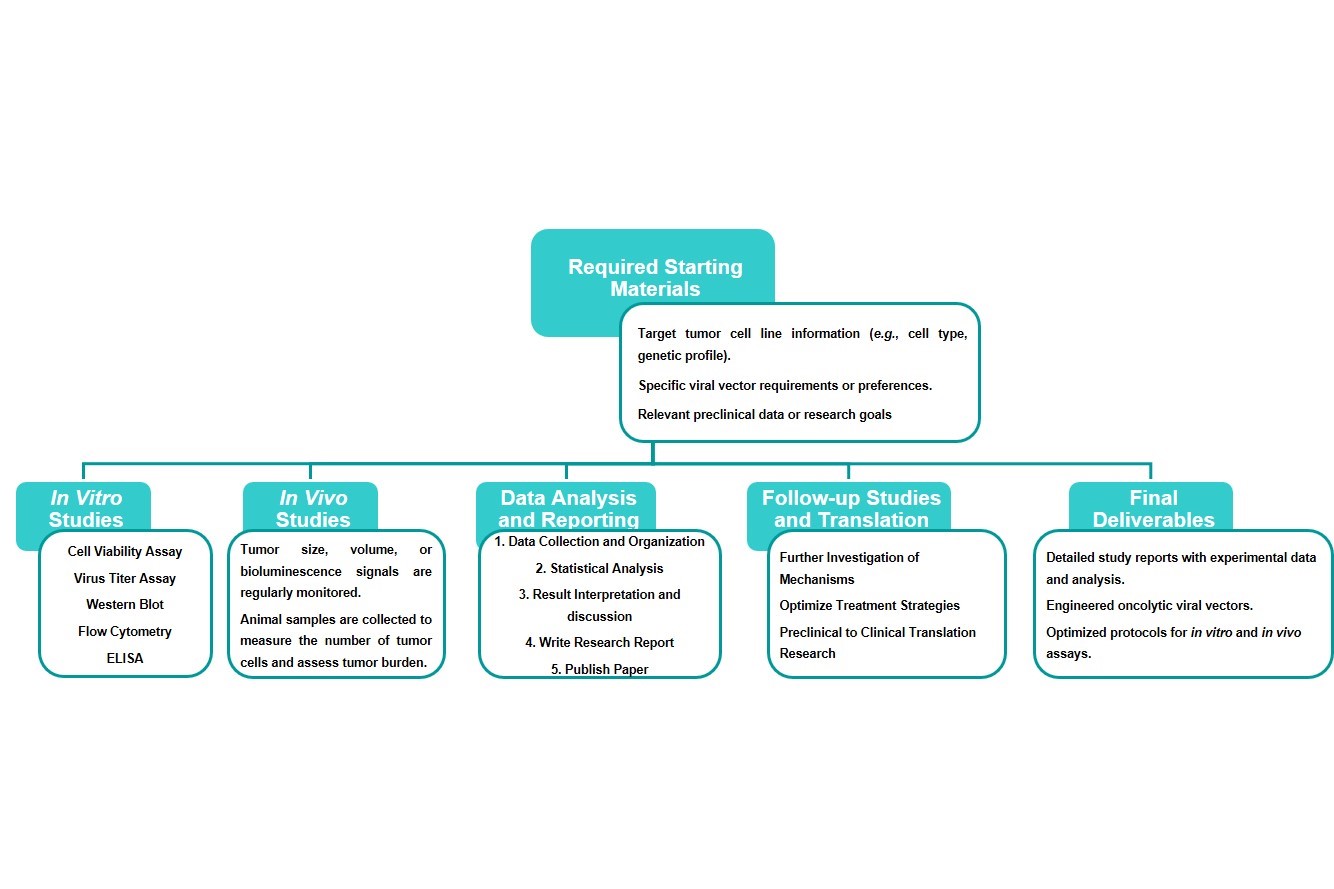
Estimated Timeframe:
Pre-requirement communication: 1-2 weeks
Design and construction of oncolytic viruses: 3-4 weeks
Mass production of oncolytic viruses: 2-3 weeks
Function and properties of oncolytic viruses in vivo and in vitro: 3-4 weeks
Results analysis and test report: 1-2 weeks
Product delivery and shipping: 2-3 weeks
Case Study
The employment of genetically-tweaked oncolytic viruses in widely adopted in vivo mouse models and in vitro cell-culture models of gynecologic malignancies has shown a substantial improvement in tumor breakdown rates. The information amassed from several published research works offers important insights into its favorable potential for gynecologic malignancies therapy.
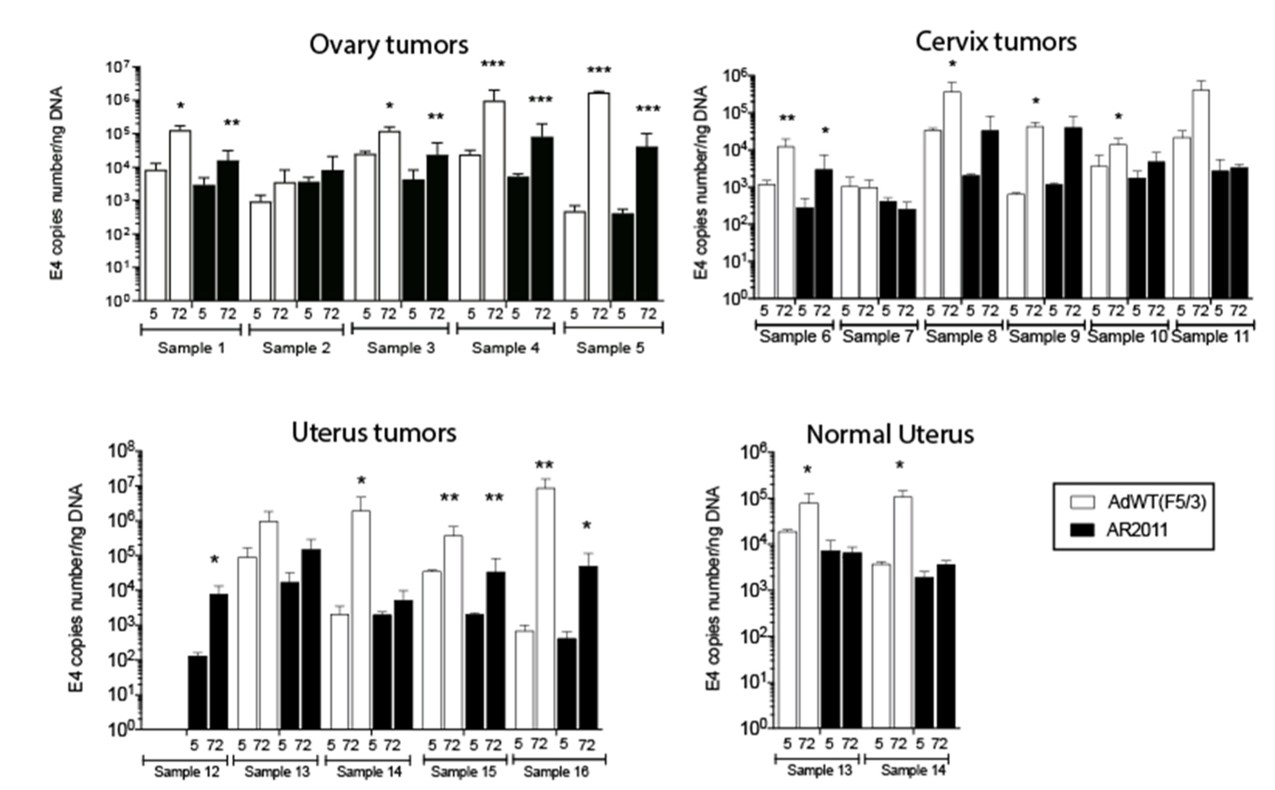 Fig.1 Detection of oncolytic virus replication in cervical cancer cells.2,4
Fig.1 Detection of oncolytic virus replication in cervical cancer cells.2,4
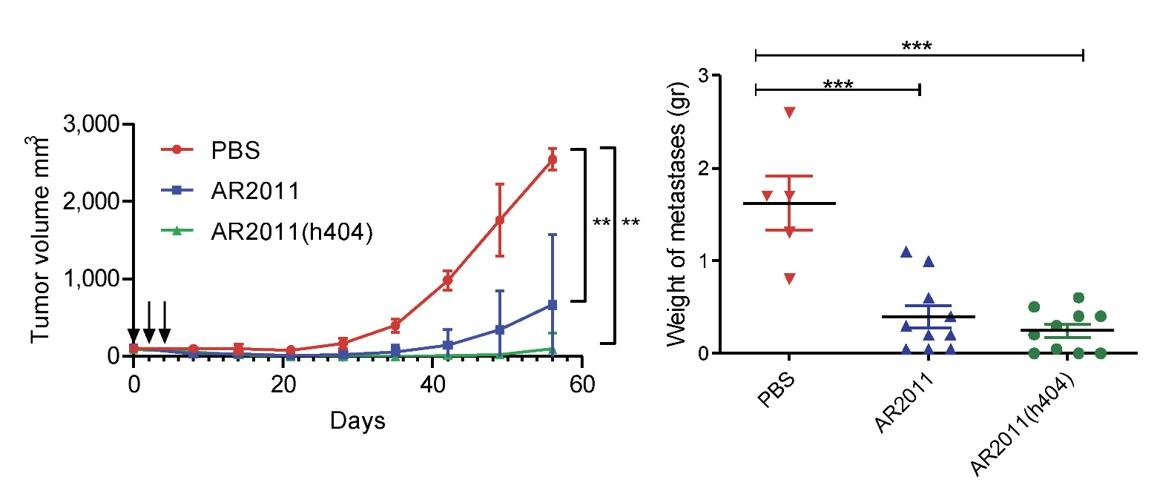 Fig.2 Oncolytic viruses control tumor growth in cervical cancer.2,4
Fig.2 Oncolytic viruses control tumor growth in cervical cancer.2,4
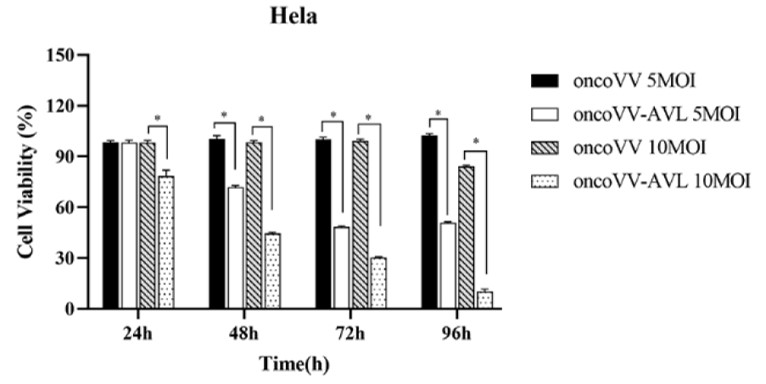 Fig.3 The effect of the oncolytic virus on the viability of cervical cancer cells was determined by MTT assay.3,4
Fig.3 The effect of the oncolytic virus on the viability of cervical cancer cells was determined by MTT assay.3,4
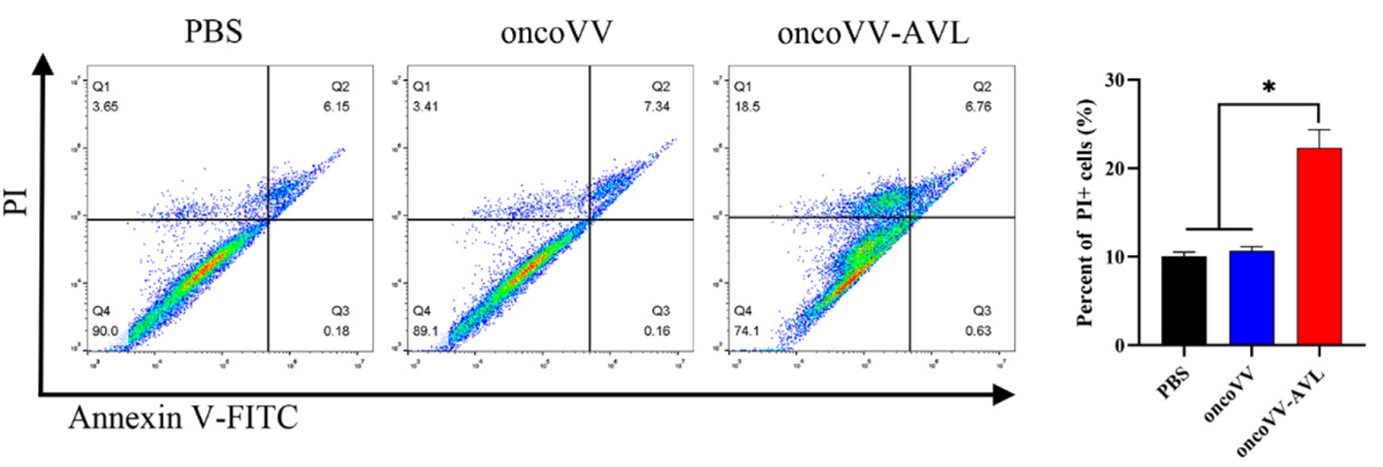 Fig.4 Flow cytometry is used to detect the ability of oncolytic viruses to induce apoptosis in cancer cells.3,4
Fig.4 Flow cytometry is used to detect the ability of oncolytic viruses to induce apoptosis in cancer cells.3,4
References
- Hu, Zongfeng, et al. "The anti-tumor efficacy of a recombinant oncolytic herpes simplex virus mediated CRISPR/Cas9 delivery targeting in HPV16-positive cervical cancer." Antiviral Research 232 (2024): 106035. DOI: 10.1016/j.antiviral.2024.106035. Distributed under Open Access license CC BY 4.0,without modification.
- Alfano, Ana, et al. "In Vitro and In Vivo Efficacy of a Stroma-Targeted, Tumor Microenvironment Responsive Oncolytic Adenovirus in Different Preclinical Models of Cancer." International journal of molecular sciences 24.12 (2023): 9992. DOI: 10.3390/ijms24129992
- Ni, Jiajun, et al. "Oncolytic vaccinia virus harboring aphrocallistes vastus lectin inhibits the growth of cervical cancer cells hela S3." Marine Drugs 19.10 (2021): 532. DOI: 10.3390/md19100532
- Distributed under Open Access license CC BY 4.0, reformat the picture.
