Genitourinary Malignancy Specific Oncolytic Virotherapy Development Service
Introduction
Genitourinary (GU) malignancies, which originate from the kidney/renal pelvis, bladder, prostate, as well as other structures such as the testis and penis, encompass malignancies of the kidney, bladder, urinary tract, prostate, testis, and penis. Throughout past research, treatment options for GU malignancies have undergone significant evolution. Besides traditional chemotherapeutic agents, multiple therapeutic modalities have come to the fore, including immune checkpoint inhibitors, molecularly targeted therapies, antibody-drug conjugate agents, and radioligand therapy. Compared with other tumor immunotherapies, oncolytic viruses (OVs) have significant advantages. These include precise targeting, fewer adverse reactions, lower likelihood of drug resistance, and a high tumor-killing effect.
The Common Types and Characteristics of Genitourinary Malignancies
Tab.1 Common types of genitourinary malignancies.
| Types of Cancer | Main manifestations |
|---|---|
| Renal cell carcinoma | RCC arises from renal tubular epithelial cells. Clinically, some patients may present with symptoms such as hematuria, lumbago, and abdominal mass. |
| Bladder cancer | Bladder cancer is the most prevalent urinary system malignancy. It's mainly characterized by painless gross hematuria, often accompanied by bladder-irritative symptoms like urinary frequency, urgency, and dysuria. |
| Prostate Cancer | Prostate cancer predominantly impacts older men and presents with indistinct early-stage manifestations. As the tumor advances, symptoms such as difficult urination, increased frequency of urination, urinary urgency, and urinary incontinence might emerge. |
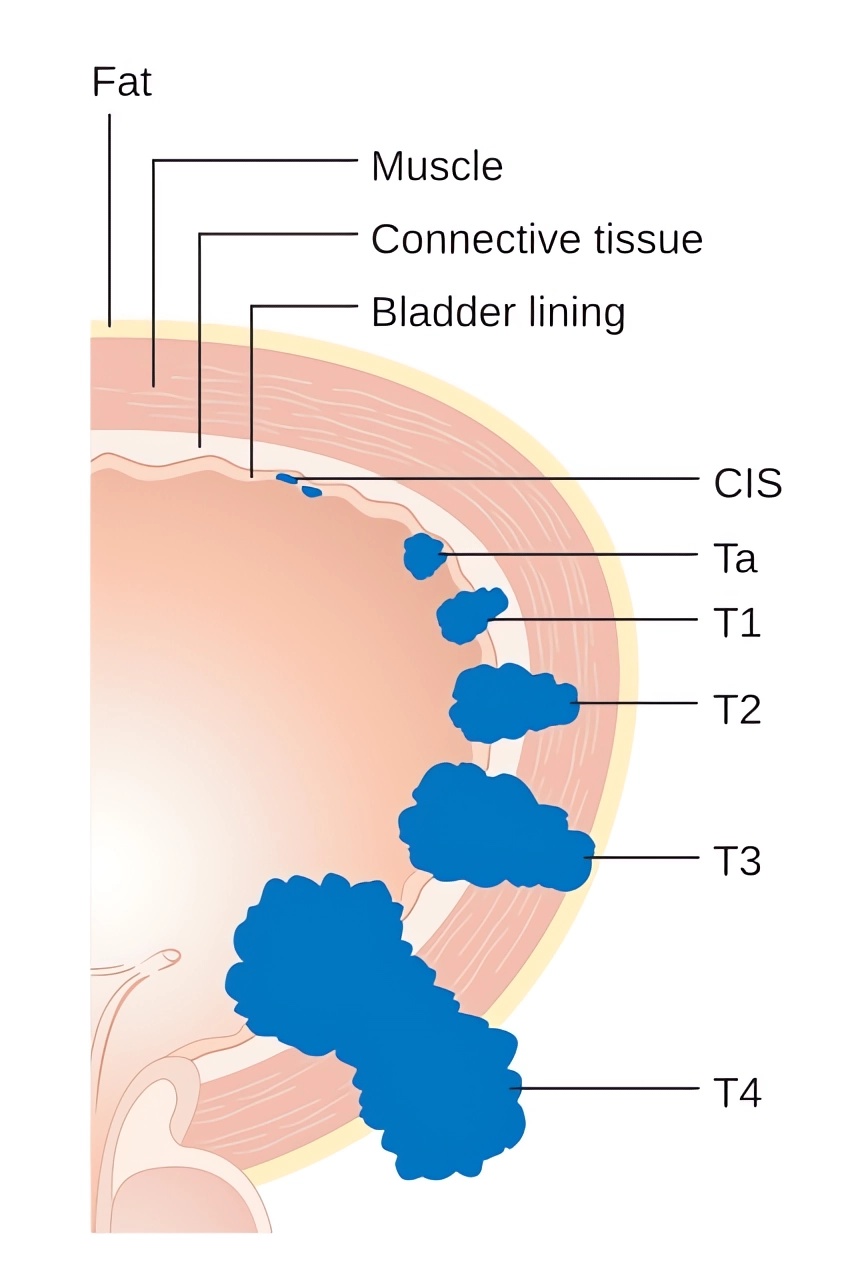 Distributed under CC BY-SA 4.0, from Wiki, without modification.
Distributed under CC BY-SA 4.0, from Wiki, without modification.
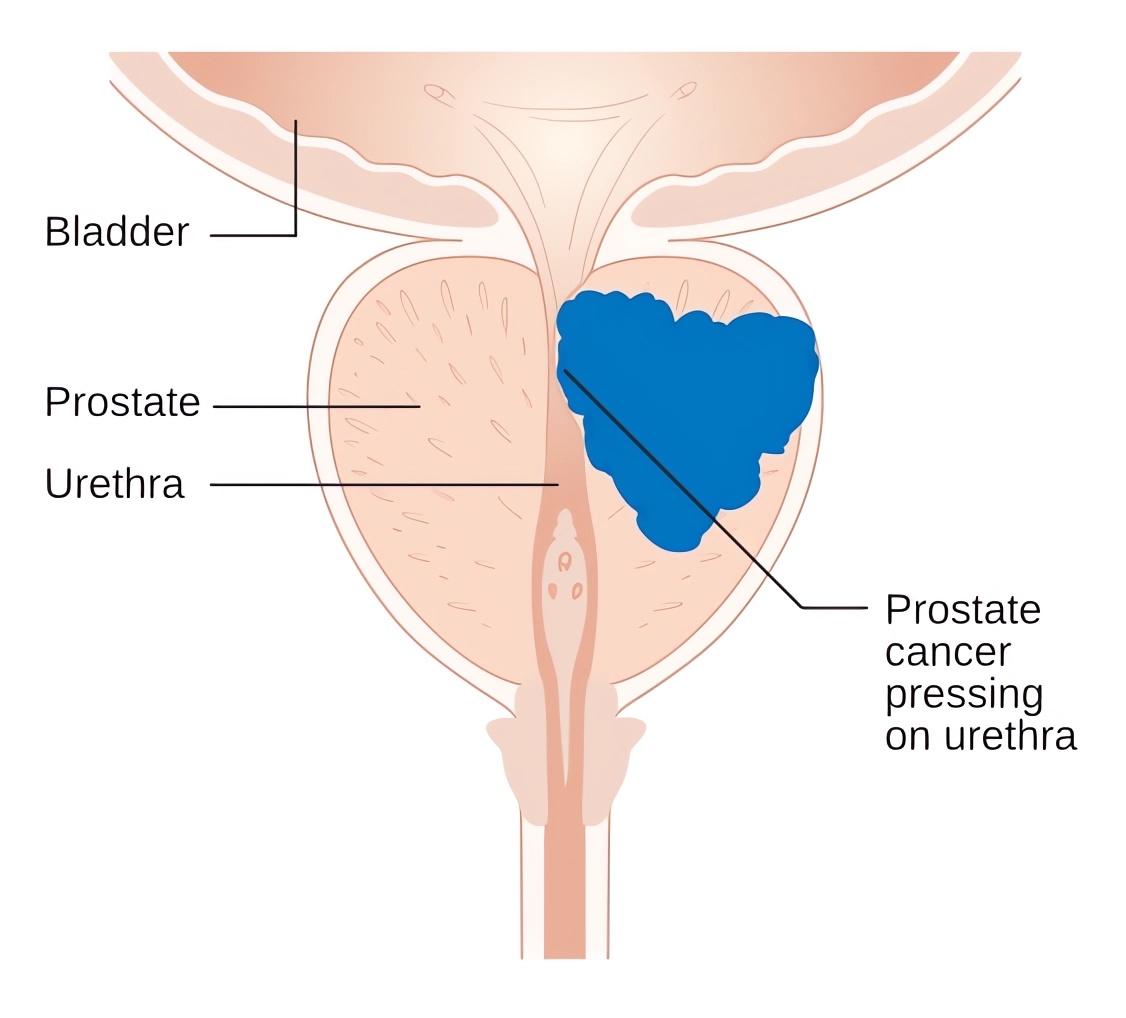 Distributed under CC BY-SA 4.0, from Wiki, without modification.
Distributed under CC BY-SA 4.0, from Wiki, without modification.
Oncolytic Virus Therapy for Genitourinary Tumors
- Oncolytic virus treatment of prostate cancer: In prostate cancer (PCa) research, LCN2 has been identified as highly expressed in tumor specimens, and it exerts regulatory effects on cell-related processes. Appropriate knockout of LCN2 can increase the susceptibility of PCa to EHDV-TAU. The oncolytic clone EHDV-TAU, isolated from the EHDV2-IBA strain, is capable of eliciting a diverse range of cell responses. Additionally, measles virus derivatives have demonstrated potential. MV-CEA can be applied for non-invasive surveillance, and MV-NIS can be utilized for imaging and therapeutic purposes. Adenoviruses engineered to express human hIL-12 have also been explored for treatment.
- Oncolytic virus treatment of bladder cancer: Intravesical immunotherapy using GM-CSF-armed oncolytic vesicular stomatitis virus has been shown to improve the prognosis of bladder cancer. A novel oncolytic vesicular stomatitis virus harboring a human GM-CSF transgene (VSVd51-hGM-CSF) is generated and evaluated as a potential bladder-sparing treatment option for aggressive bladder cancer. OV can be administered intravenously, intravesical, and intratumoral in the treatment of bladder cancer. Infection and direct oncolytic results in the release of DAMPs, PAMPs, TAA, and various cytokines. These molecules are responsible for drawing in and rousing APC including dendritic cells (DC), natural killer (NK) cells, and other immune cells to where the infection occurs.
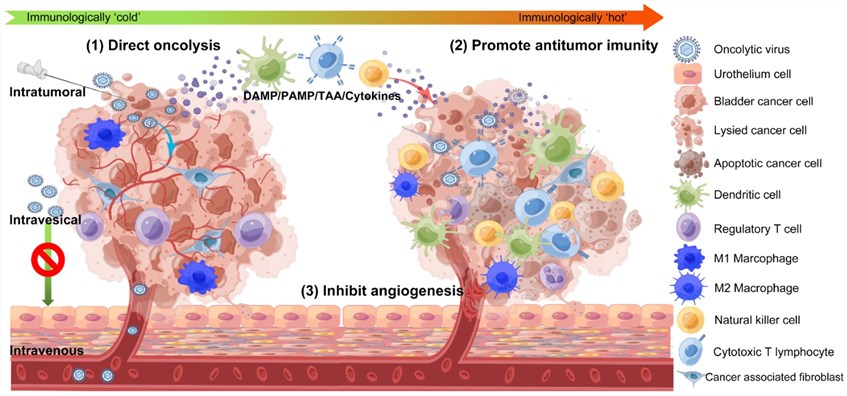 Fig.1 Mechanisms of oncolytic virus therapy for prostate tumors.1
Fig.1 Mechanisms of oncolytic virus therapy for prostate tumors.1
Tab.2 Preclinical studies on oncolytic viruses for Bladder cancer.1
| Parent Virus | Oncolytic Virus | Virus Description |
|---|---|---|
| Adenovirus | Ad5-d55k | Serotype-5 adenoviruses that have been debilitated for selective replication within p53-deficient (Ad5-d55K) cells |
| ONYX-015 | An adenovirus with its E1B 55 - kDa gene deleted and engineered to replicate exclusively within and lyse cancer cells deficient in p53 | |
| CG0070 | Adenoviruses armed with GM-CSF. | |
| Onco(Ad).RGD-hTERT-TRAIL | An RGD-fiber modified oncolytic adenovirus carrying TRAIL gene and EGFP | |
| Alphavirus | M1 | A Getah-like virus with a positive single-strand RNA genome |
| Coxsackievirus | CVA21 | A novel ICAM-1 targeted immunotherapeutic virus |
| Nv1066 | An attenuated mutant of HSV - 1 which generates green fluorescent protein and is devoid of the viral genes ICP0 and ICP4. | |
| HSV-1 | HF10 | A significantly attenuated, replication-capable variant of HSV-1 |
| oHSV-1 | A HSV-1 mutant that expresses endogenous miR-124 and miR-143 | |
| HSV-2 | FusOn-H2 | A mutant of HSV-2, from which the protein kinase domain has been deleted and which specifically targets BC cells through activation of the Ras signaling system |
| Pseudorabies virus | YP2 | A modified pseudorabies virus that has the genes for HSV-1 thymidine kinase and glycoprotein D. |
| NDV | LaSota Strain | Recombinant lentogenic NDV LaSota strain |
| VV | ΔF4L VV | F4L-deleted vaccinia virus |
| VSV | VSVd5-hGM-CSF | VSV with the human GM-CSF transgene inserted into its genome |
| Reovirus | AV3 | A mutant Delta51M variant |
Strategies for oncolytic viral gene editing
To further enhance the therapeutic efficacy and minimize potential adverse impacts, the functional optimization and attenuation of immunogenicity or toxicity of oncolytic viruses have emerged as pivotal research foci. A proven strategy entails the genetic engineering of oncolytic viruses targeting specific genes.
- TP53: TP53 gene mutations or deletions are prevalent in diverse genitourinary tumors, including renal cell carcinoma and bladder cancer. Gene therapy that fixes or adds the normal TP53 gene can bring back its role in stopping tumors. This approach can trigger the programmed death of tumor cells and also prevent tumor cells from growing and multiplying.
- RB: Mutations or deletions in the RB gene disrupt cell-cycle regulation, predisposing to abnormal tumor cell proliferation. In genitourinary tumors like prostate cancer, RB gene abnormalities are prevalent. Editing the RB gene or promoting its overexpression can restore normal cell-cycle control and impede tumor cell growth.
- PSA: The prostate-specific antigen (PSA) gene is specifically expressed in prostate cancer cells. Engineering a PSA/hTERT-driven oncolytic virus can downregulate the expression of CD24, CD44, and PSCA in cancer cells and tissues, decrease cancer tissue weight, inhibit angiogenesis within cancer tissues, and modulate the immune response in tumor tissues.
- HER2: Human epidermal growth factor receptor-2 (HER-2) is overexpressed in certain urogenital tumors, like bladder cancer. The HER2 gene is a good target for gene therapy. Stopping the HER2 gene from working or blocking the way it sends signals can slow down the growth and stop the spread of tumor cells. It also can help the immune system better identify and kill tumor cells.
- IL12: IL-12 promotes the proliferation and activation of T cells, NK cells, and other immune cells. Engineering an oncolytic virus to express the IL12 can enhance the body's anti-tumor immune response. By infecting tumor cells, it improves the ability of immune cells to kill tumor cells.
Workflow
Estimated Timeframe:
Pre-requirement communication: 1-2 weeks
Design and construction of oncolytic viruses: 3-4 weeks
Mass production of oncolytic viruses: 2-3 weeks
Function and properties of oncolytic viruses in vivo and in vitro: 3-4 weeks
Results analysis and test report: 1-2 weeks
Product delivery and shipping: 2-3 weeks
Case Study
The employment of genetically-altered oncolytic viruses in commonly utilized in vivo murine models and in vitro cell-culture models of genitourinary malignancies has yielded a significant improvement in tumor-elimination effectiveness. Findings retrieved from an array of research reports offer crucial outlooks on its promising potential for the treatment of genitourinary malignancies.
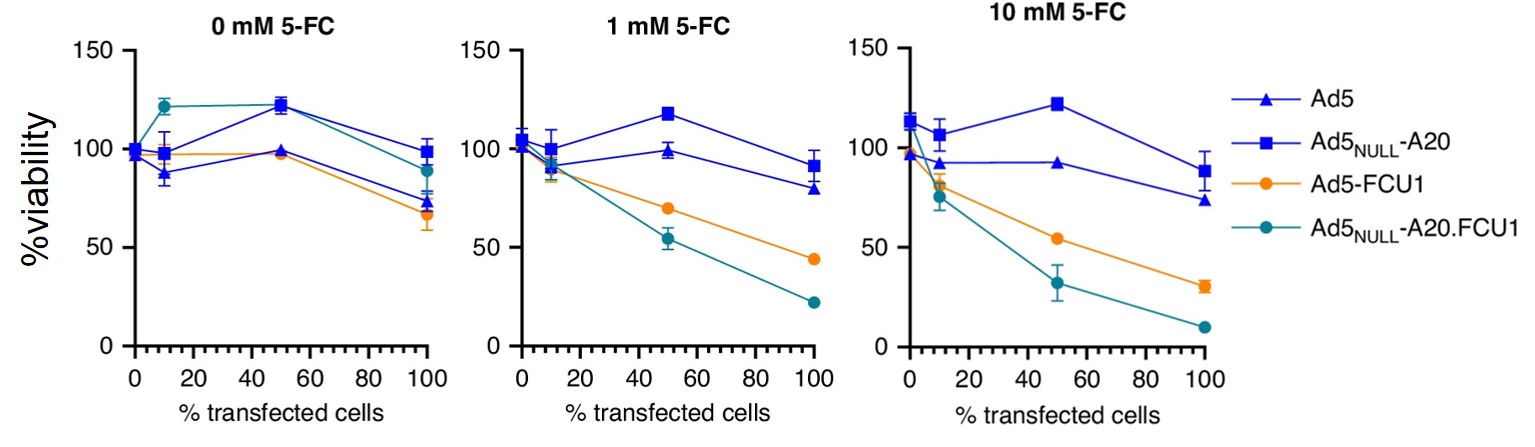 Fig.2 The activity of tumor cells at different drug concentrations is detected.2
Fig.2 The activity of tumor cells at different drug concentrations is detected.2
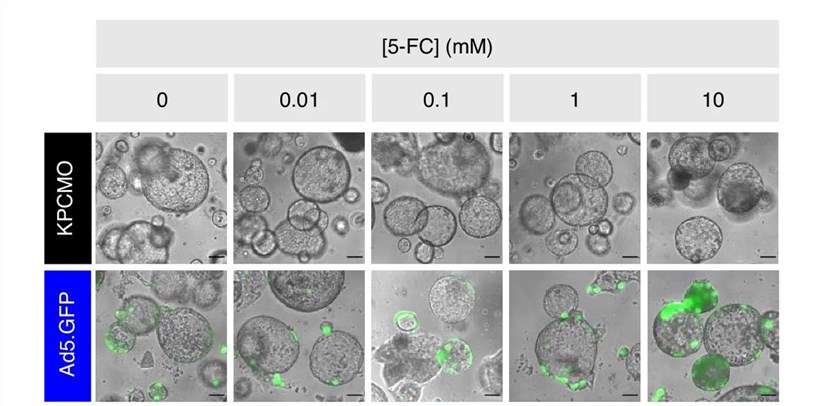 Fig.3 Fluorescence microscopy is used to visualize the replication of oncolytic viruses with GFP in tumor organoids.2
Fig.3 Fluorescence microscopy is used to visualize the replication of oncolytic viruses with GFP in tumor organoids.2
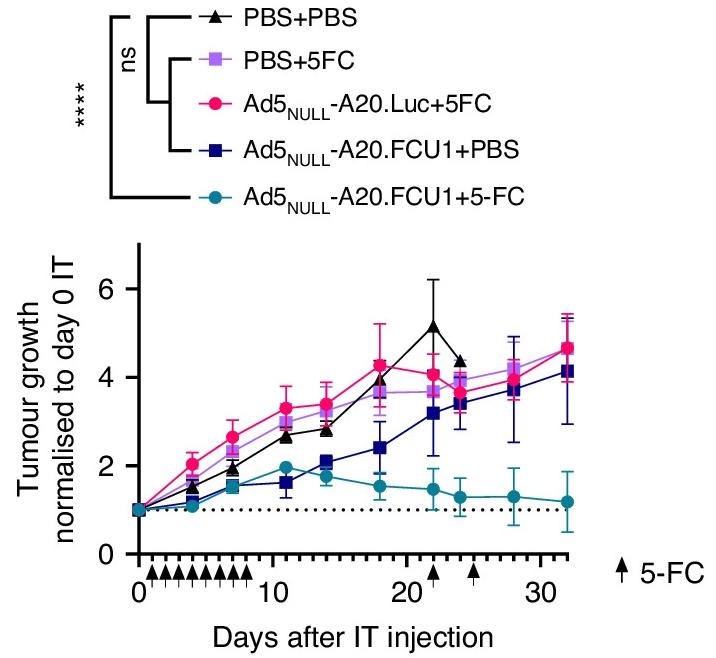 Fig.4 Tumor growth after injection of oncolytic virus.2
Fig.4 Tumor growth after injection of oncolytic virus.2
References
- Hu, Henglong, et al. "Oncolytic viruses for the treatment of bladder cancer: advances, challenges, and pro spects." Journal of Clinical Medicine 11.23 (2022): 6997. DOI: 10.3390/jcm11236997. Distributed under Open Access license CC BY 4.0, without modification.
- Badder, Luned M., et al. "The αvβ6 integrin specific virotherapy, Ad5NULL-A20. FCU1, selectively delivers potent "in-tumour" chemotherapy to pancreatic ductal adenocarcinoma." British Journal of Cancer 131.10 (2024): 1694-1706. DOI: 10.1038/s41416-024-02869-3. Distributed under Open Access license CC BY 4.0, reformat the picture.
