Oncolytic Vesicular Stomatitis Virus Construction Service
Vesicular stomatitis virus (VSV) has been utilized for the elimination of tumor cells since its capacity to inhibit melanoma growth in murine models is discovered. VSV is characterized by a short replication cycle, minimal damage to normal cells, broad tissue tropism, adaptability to hypoxic environments, a small viral genome, and ease of modification. In an effort to bolster the safety of oncolytic VSV and concurrently augment its capacity for carrying therapeutic transgenes, Creative Biolabs has set up an all-encompassing platform named OncoVirapy™. This platform is dedicated to devising, engineering, and fabricating the most potent recombinant VSV and associated products.
Introduction of Vesicular Stomatitis Virus
VSV, belonging to the Vesiculovirus genus within the Rhabdoviridae family, is an enveloped, single-stranded negative-sense RNA virus. Its genome, 11,161 nucleotides in length, encodes five proteins in the order from the 3' to the 5' end: nucleocapsid protein (N), phosphoprotein (P), matrix protein (M), glycoprotein (G), and large polymerase protein (L).
VSV can be classified into two serotypes, with the Indiana (IN) and New Jersey (NJ) strains serving as representatives. According to antigen cross-reactivity, the IN subtype is subdivided into IN1, IN2 (Cocal), and IN3 (Alagoas VSV).
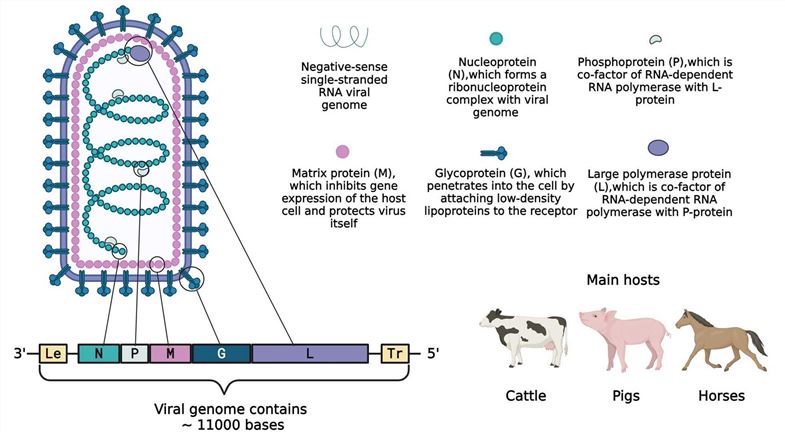 Fig.1 The protein encoded by the VSV gene and the main host.1
Fig.1 The protein encoded by the VSV gene and the main host.1
Functions of proteins encoded by the VSV genome
- Matrix protein (M): The matrix protein (M) participates in viral assembly, suppresses the gene expression in host cells, and defends the virus itself against the native immune response of the host organism.
- Glycoprotein (G): The Glycoprotein on the surface of VSV is responsible for the attachment and entry of the virus into cells via the LDLR, and participates in the uptake of cholesterol molecules, which contributes to the broad viral tropism of VSV.
- Large polymerase protein (L)/Phosphoprotein (P): L and P are the co-factors of RNA-dependent RNA polymerase
- Nucleocapsid protein (N): Encapsulating the viral genome to form the ribonucleoprotein complex (RNP).
Genetic Modifications of Oncolytic VSV Vectors
-
Vaccinia Virus Virulence Genes
- Attenuated recombinant VSV with mutations encoded at position 51 of the M protein: Through the design of a substitution (M51R) or deletion (rVSVΔM51) at position 51 of the M protein, the inhibitory effect of VSV on the innate antiviral response was attenuated. Meanwhile, the capacity for selective replication within cancer cells was maintained, and the risk of neurotoxic effects was mitigated.
- C-terminal truncation of the G protein (GΔ28) substantially impacts the efficiency of virus budding and packaging processes. When VSV infects target cells, the G glycoprotein thereof induces endocytosis, thereby facilitating the virus's entry into the cells.
- Virus pseudotyping: The tropism of VSV for malignant tumors can be augmented by encoding heterologous glycoproteins. For instance, substituting the VSV G protein with the F and H proteins of the Measles Virus (MV) led to the complete regression of hepatocellular carcinoma cells2. Moreover, the VSV G protein can be replaced by the glycoprotein of Lymphocytic Choriomeningitis Virus (LCMV) or by the glycoprotein of Sindbis Virus.
- Insertion of Additional Exogenous Genes
By inserting various modified sequences between G and L, the tumor selectivity and safety of VSV can be enhanced. Typically, transgenes related to cytokines, chemokines, pro-apoptotic genes, tumor-associated antigens (TAA), immune checkpoint inhibitors (ICI), and other types of transgenes can be introduced.
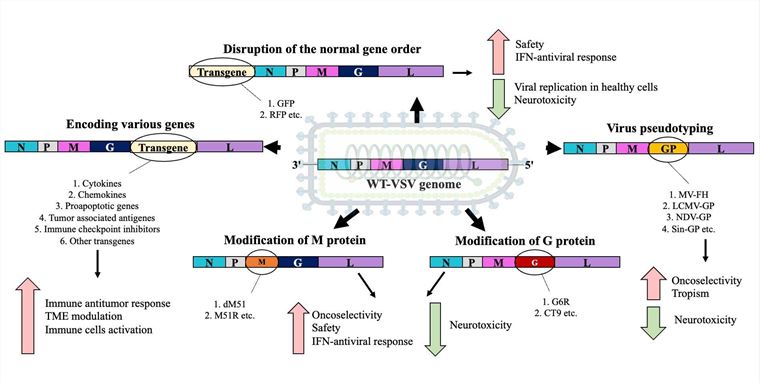 Fig.2 The common strategies for VSV modifications.1
Fig.2 The common strategies for VSV modifications.1
- The p53 gene mediates DNA repair, cell senescence, apoptosis, and immune activation by regulating the cell-cycle signaling pathway, thereby effectively inhibiting the occurrence and development of tumors. Heiber et al. integrated the mouse p53 gene into VSV and constructed a recombinant VSV with high-level expression of the p53 gene product. This recombinant VSV demonstrated higher viral replication efficiency and enhanced oncolytic potency.
- The integration of suicide genes not only augments the cytotoxic effect of VSV on infected tumor cells but also endows VSV with the ability to eliminate uninfected tumor cells via gap junctions between cells. The thymidine kinase/ganciclovir (TK/GCV) and the cytosine deaminase/uracil phosphoribosyl transferase (CD/UPRT) suicide gene system are extensively applied in the realm of gene therapy.
Featured Services and Construction Workflow
We provide a comprehensive range of VSV services based on our advanced OncoVirapy™ platform, including but not limited to the following items:
- Select the GOI (encoding antibodies, cytokines, chemokines or immune checkpoint inhibitors, etc.) or the genes plan to be deleted for restriction mapping and sequencing verification
- Functional validation of transgenes
- Expression cassette design with the most appropriate promoter to guarantee the high-level expression
- Viral gene engineering (single gene or different genes combination)
- Different construction strategies (homologous recombination, BAC system, etc.)
- VSV amplification, purification, titering services
- VSV whole genome sequencing (WGS) service
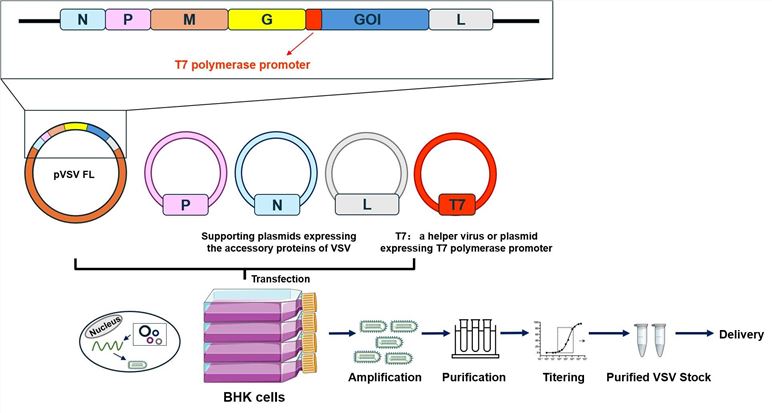 Fig.3 Workflow for Creative Biolabs construction of oncolytic vesicular stomatitis virus.
Fig.3 Workflow for Creative Biolabs construction of oncolytic vesicular stomatitis virus.
Cases of VSV Genetic Modifications
Tab.1 Oncolytic vesicular stomatitis virus undergoing clinical trials
| VSV Strain | Tumor Selectivity | Route of Administration | Phase |
|---|---|---|---|
| VSV-IFNbetaTYRP1 | Melanoma stage Ⅲ-Ⅳ | IT, IV | Ⅰ |
| VSV-IFN-beta | Liver cancer | IT | Ⅰ |
| VSV-hIFNbeta-NIS | Metastatic or Recurrent Endometrial Cancer | IV | Ⅰ |
| Acute Myeloid Leukemia or Lymphoma | IV | Ⅰ | |
| Malignant Solid Tumor | IT | Ⅰ | |
| VSV-GP128 | Stage Ⅳ colorectal cancer | IV | Ⅰ |
| VSV (Revottack) | Advanced malignant solid tumors | IV | Ⅰ |
| VSV-GP154 | Pancreatic cancer | IV (injection) | Ⅰ |
Creative Biolabs provides a variety of custom gene editing services for oncolytic vesicular stomatitis virus, featuring high precision and comprehensive technical support. Moreover, we offer pre-clinical evaluation services (in vivo or in vitro) for the modified oncolytic agents to accelerate the client's research progress.
References
- Zinovieva, Margarita, et al. "Oncolytic Vesicular Stomatitis Virus: Optimisation Strategies for Anti-Cancer Therapies." Frontiers in Bioscience-Landmark 29.11 (2024): 374. Distributed under Open Access license CC BY 4.0, without modification.
- Nagalo, Bolni Marius, et al. "Oncolytic virus with attributes of vesicular stomatitis virus and measles virus in hepatobiliary and pancreatic cancers." Molecular Therapy-Oncolytics 18 (2020): 546-555.
