Mesothelioma Specific Oncolytic Virotherapy Development Service
Accelerate Your Mesothelioma Virotherapy Development!
Are you currently facing the challenges of limited efficacy with conventional mesothelioma therapies and the complexities of developing novel treatment strategies? Creative Biolabs' Oncolytic Virotherapy Development for Mesothelioma service helps you accelerate the development of innovative cancer treatments through our comprehensive OncoVirapy™ platform and extensive expertise in oncolytic virus engineering.
How Creative Biolabs Oncolytic Virotherapy Can Assist Your Project
This service offers clients customized oncolytic virus-based approaches for the development of mesothelioma treatments. Creative Biolabs utilizes cutting-edge technologies to engineer, build, and verify oncolytic viruses that have improved tumor-targeting capabilities and greater therapeutic promise.
Deliverables:
- Genetically engineered oncolytic viruses are designed to target mesothelioma cells.
- Comprehensive in vitro and in vivo validation data demonstrating the efficacy and safety of the developed viruses.
- Detailed reports outlining the construction process, characterization data, and experimental results.
[Discover How We Can Help - Request a Consultation]
Workflow
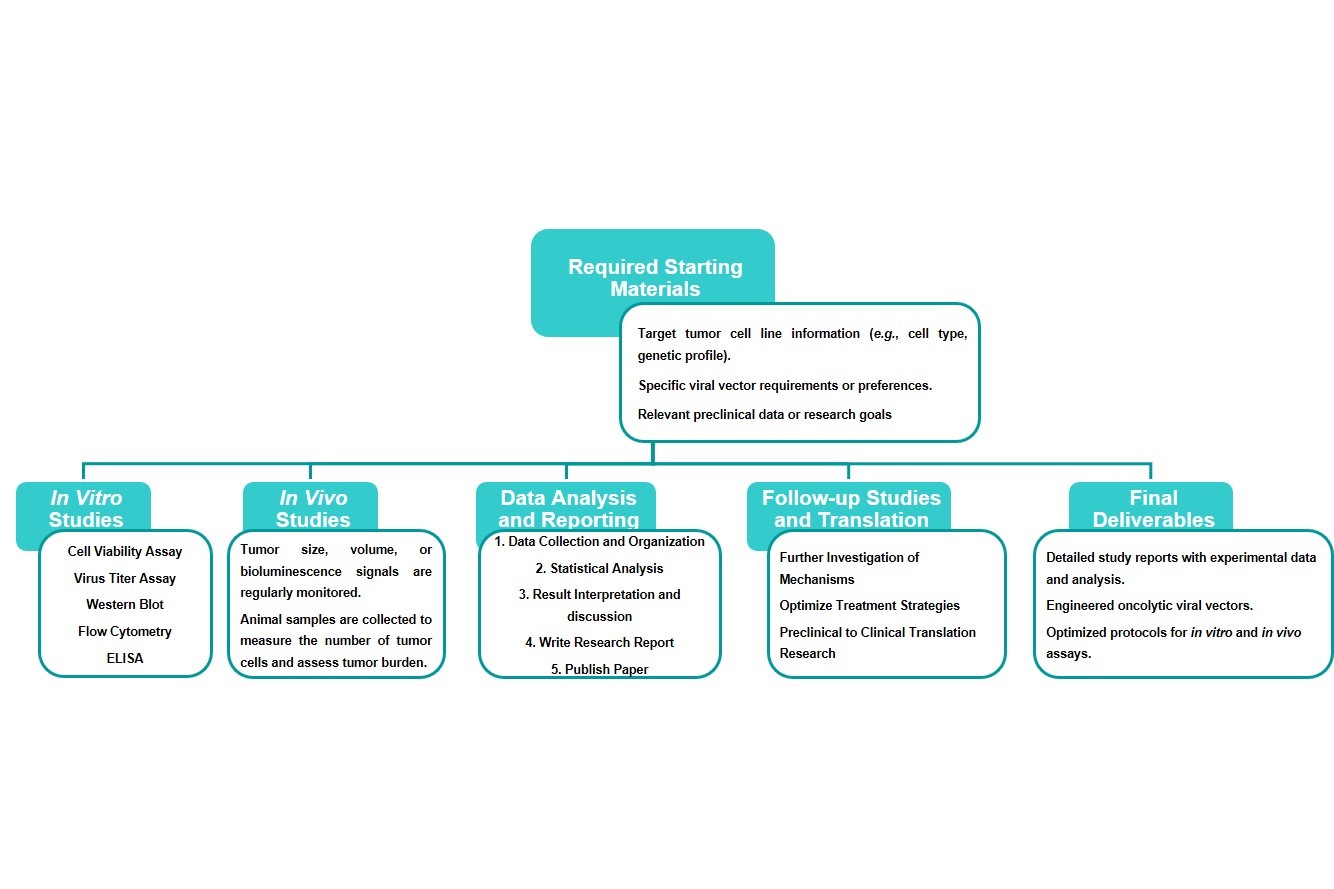
Estimated Timeframe:
Pre-requirement communication: 1-2 weeks
Design and construction of oncolytic viruses: 3-4 weeks
Mass production of oncolytic viruses: 2-3 weeks
Function and properties of oncolytic viruses in vivo and in vitro: 3-4 weeks
Results analysis and test report: 1-2 weeks
Product delivery and shipping: 2-3 weeks
Highlight
- Comprehensive OncoVirapy™ Platform: Creative Biolabs provides a suite of tools and technologies for every stage of oncolytic virus development.
- Expert Scientific Team: Our scientists have extensive experience in virology, gene therapy, and cancer biology.
- Customized Solutions: We tailor our services to meet the specific needs of each client's project.
- Proven Track Record: Creative Biolabs has a history of successfully developing oncolytic virus-based therapeutics for various cancer types. Published Data supports our expertise.
[Experience the Creative Biolabs Advantage - Get a Quote Today]
What We Can Offer
Creative Biolabs offers biology experts a comprehensive suite of oncolytic virotherapy development services, including:
- Custom Oncolytic Virus Design and Construction: We engineer viruses tailored to your specific mesothelioma targets, incorporating the latest advancements in vector technology and therapeutic gene delivery.
- End-to-End Preclinical Development: From in vitro studies to in vivo efficacy and safety testing in relevant mesothelioma models, we provide a complete preclinical development pathway.
- GMP-Grade Virus Production: We offer scalable production of oncolytic viruses under GMP conditions, facilitating your transition to clinical trials.
- Regulatory Consultation: Our regulatory experts provide guidance and support to navigate the complex regulatory landscape of oncolytic virus-based therapeutics.
- Collaborative Research Partnerships: We foster long-term collaborations, working closely with you to achieve your research and development goals.
Case Study
The utilization of genetically modified oncolytic viruses in commonly employed in vivo mouse models and in vitro mesothelioma cell line models has led to a significant enhancement in the efficacy of tumor cell destruction. Findings derived from numerous published research reports offer crucial insights into its promising potential for mesothelioma treatment.
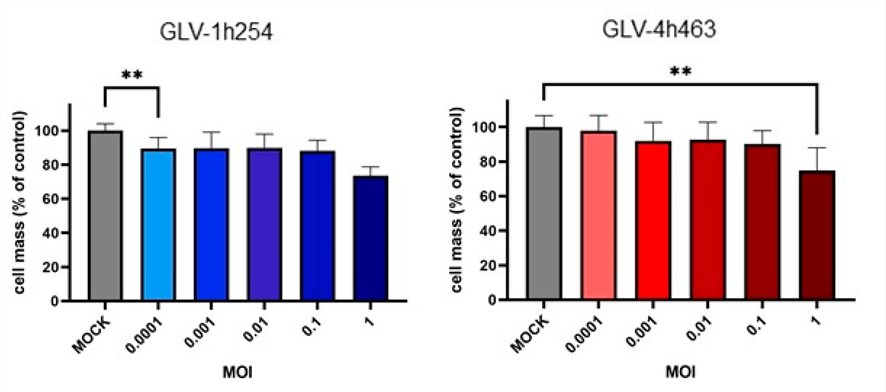 Fig.1 Effect of oncolytic viruses on mesothelioma cell activity.1,4
Fig.1 Effect of oncolytic viruses on mesothelioma cell activity.1,4
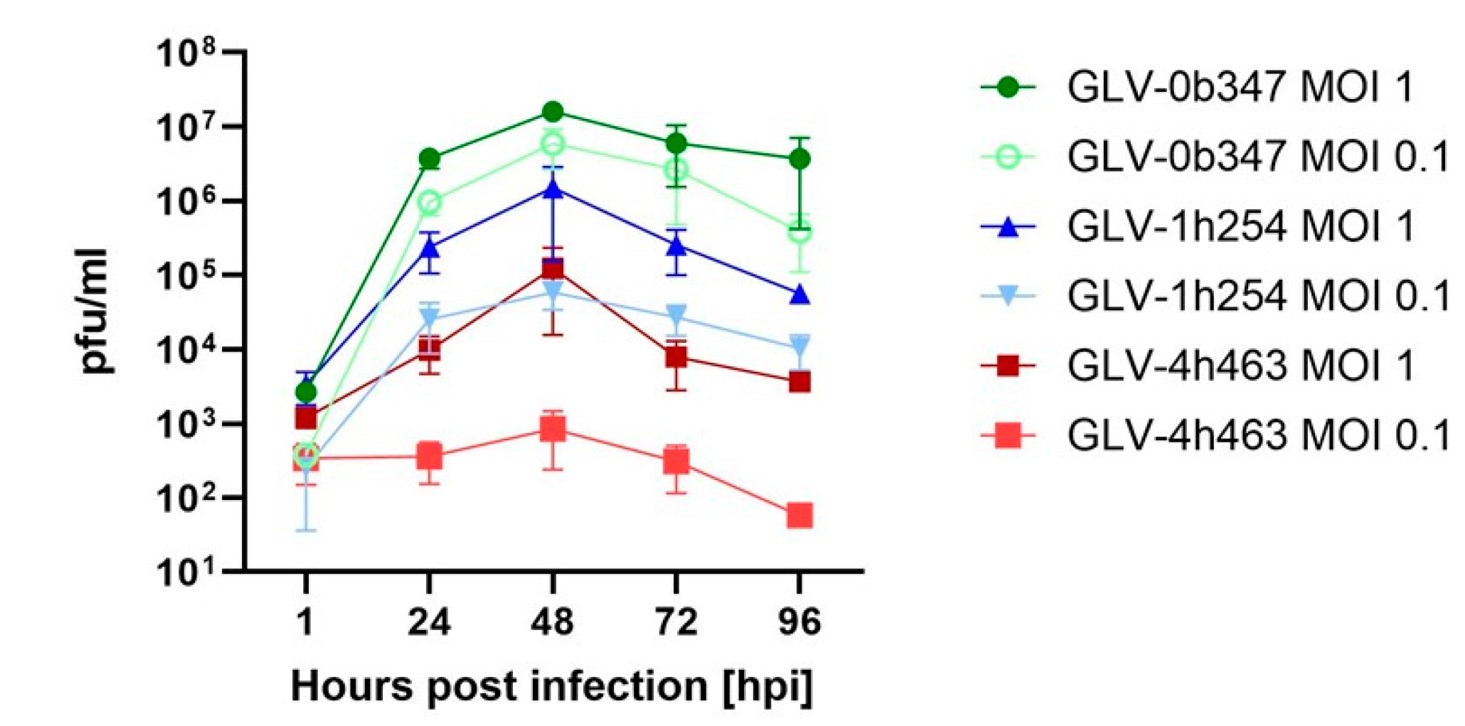 Fig.2 Detection of the replication capacity of oncolytic viruses in mesothelioma cells.1,4
Fig.2 Detection of the replication capacity of oncolytic viruses in mesothelioma cells.1,4
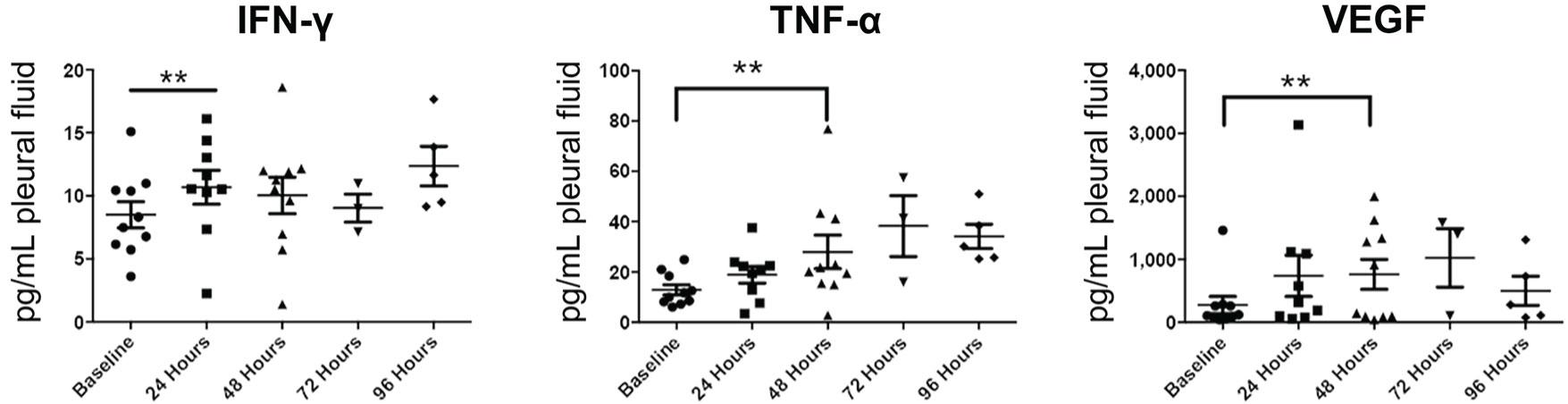 Fig.3 Oncolytic viruses promote cytokine secretion.2,4
Fig.3 Oncolytic viruses promote cytokine secretion.2,4
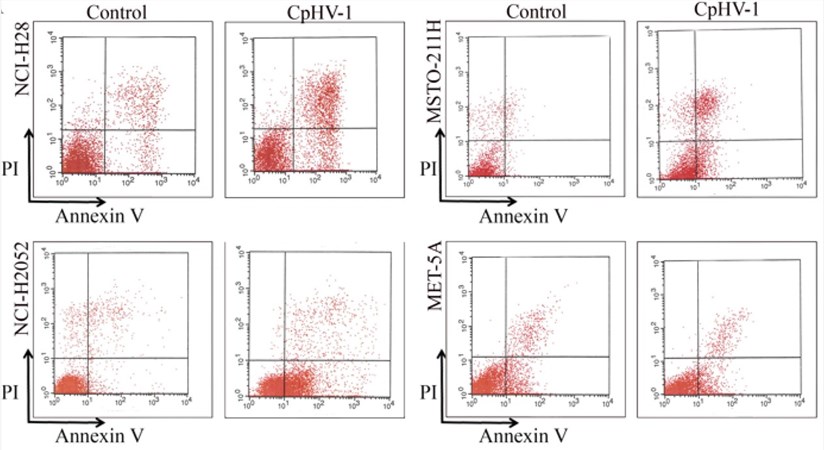 Fig.4 Flow cytometry was used to detect the effect of oncolytic virus treatment on tumor cell apoptosis.3,4
Fig.4 Flow cytometry was used to detect the effect of oncolytic virus treatment on tumor cell apoptosis.3,4
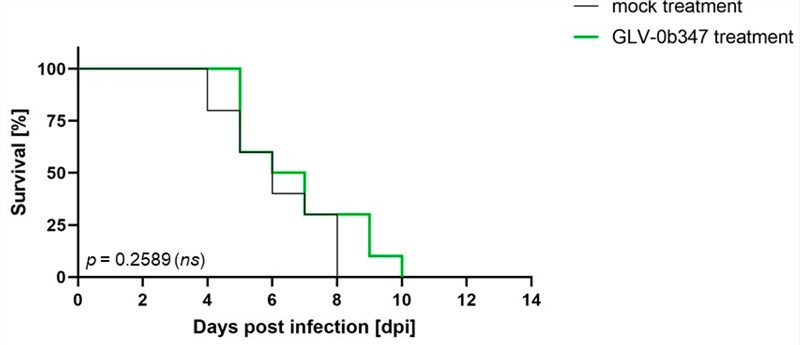 Fig.5 Survival curves of tumor-bearing mice.1,4
Fig.5 Survival curves of tumor-bearing mice.1,4
Customer Reviews:
-
"Using Creative Biolabs' Oncolytic Virotherapy Development service in our research has significantly improved the efficiency of our in vivo studies."
[10 Months], J**n
-
"Creative Biolabs' expertise in virus engineering and validation was crucial for advancing our oncolytic virus candidate to the next stage of development. The data package was comprehensive and high quality."
[12 Months], Dr. P**r
-
"The customized approach and collaborative spirit of the Creative Biolabs team made them an ideal partner for our mesothelioma research program. Their platform significantly accelerated our timeline."
[9 Months], E**y
Introduction of Mesothelioma
Malignant pleural mesothelioma (MPM) is a highly invasive cancer, and currently, viable treatment alternatives are scarce. MPM originates from mesothelial cells, predominantly the pleural membrane enveloping the lungs. A strong link exists between this cancer type and previous asbestos exposure.
Oncolytic Virotherapy Development for Mesothelioma Service
- HSV-1: Recombinant HSV-1 has been studied in malignancies, and T-VEC, a modified HSV-1, is the only FDA-approved OV for melanoma. Preclinical studies show that oncolytic herpesviruses (G207, NV1020, and NV1066) are cytotoxic to mesothelioma cell lines. Deletion of the G207γ134.5 gene and inactivation of the ICP6 gene further diminished replication in nonreplicating tissues. NV1020, initially devised as an HSV vaccine, encodes ICP0, ICP4, latency-associated transcripts, one γ134.5 copy, and has a UL24 deletion, all causing virulence loss. NV1066 has single-copy deletions of ICP0, ICP4, and γ134.5, with GFP added for viral imaging.
- These viruses were tested in vitro against 11 different MPM cell lines, covering all MPM histological subtypes (epithelioid, sarcomatous, biphasic, and mixed). All three viruses showed cytotoxicity to each cell line, even at low multiplicity of infection. In MPM mouse models, NV1066 treatment reduced tumor burden and improved survival.
- Vaccinia Virus: Replication-competent vaccinia virus GLV-1h68 has been shown to replicate in and lyse mesothelioma cell lines in vitro. In murine models, intrapleural delivery of the virus reduced tumor burden and improved survival.
- The MV-mIFNβ-NIS synthesized by inserting IFN-β and NIS genes into the measles virus can be used as a potential treatment for mesothelioma. The results showed that the oncolytic virus could increase the infiltration of immune cells around the tumor and reduce angiogenesis.
- An oncolytic adenovirus (Ad5-D24-GM-CSF) was modified for tumor selectivity and a transgene for GM-CSF was inserted to enhance the immune response. Its clinical treatment results prolong the survival time of patients, without adverse reactions, and have good safety.
References
- Yurttas, Can, et al. "Efficacy of different oncolytic vaccinia virus strains for the treatment of murine peritoneal mesothelioma." Cancers 16.2 (2024): 368. DOI: 10.3390/cancers16020368
- Chintala, Navin K., et al. "Correlative analysis from a phase I clinical trial of intrapleural administration of oncolytic vaccinia virus (Olvi-vec) in patients with malignant pleural mesothelioma." Frontiers in Immunology 14 (2023): 1112960. DOI: 10.3389/fimmu.2023.1112960
- Forte, Iris Maria, et al. "The oncolytic caprine herpesvirus 1 (CpHV-1) induces apoptosis and synergizes with cisplatin in mesothelioma cell lines: a new potential virotherapy approach." Viruses 13.12 (2021): 2458. DOI: 10.3390/v13122458
- Distributed under Open Access license CC BY 4.0, Some of the pictures were edited and reformatted.
