Immune Checkpoint Inhibitor & Oncolytic Virotherapy Combination Therapy Development Service
Accelerate Your Immuno-Oncology Research!
How Creative Biolabs Can Assist Your Project
Oncolytic Virotherapy Development for Combination Therapy with Immune Checkpoint Inhibitors can speed up your immuno-oncology research. A lot of researchers encounter difficulties in optimizing cancer immunotherapy, for example, low response rates and tumor resistance to immune checkpoint inhibitors. Creative Biolabs' Oncolytic Virotherapy Development for Combination Therapy with Immune Checkpoint Inhibitors tackles these problems. It improves the effectiveness of immune checkpoint inhibitors and gets around these limitations by utilizing targeted viral delivery and immune regulation.
Creative Biolabs provides comprehensive solutions to integrate oncolytic virotherapy with immune checkpoint inhibitors, maximizing therapeutic potential.
Workflow
| Required Starting Materials | Oncolytic Virus Design and Construction |
|---|---|
|
We design and engineer oncolytic viruses to selectively target tumor cells and express or deliver immune-modulatory transgenes, including those that enhance ICI activity. |
| In Vitro Evaluation | In Vivo Preclinical Studies |
| The oncolytic viruses are tested in vitro using tumor cell lines to assess their oncolytic activity, transgene expression, and synergistic effects with ICIs. This includes assays such as viral titer determination, and cell viability assays. | Selected oncolytic viruses are preclinically evaluated in animal models when combined with immune checkpoint inhibitors (ICIs). The assessment focuses on aspects like tumor regression, T cell activation, and overall survival. |
| In Vivo Efficacy Studies | Immunological Assays |
| The efficacy of combined therapy was evaluated by establishing an in vivo model, monitoring tumor growth, assessing T cell infiltration into the tumor, and analyzing overall survival. | Flow cytometry, Elisa, immunohistochemistry, Western Blot, and other immunological experiments were used to elucidate the mechanism of action and evaluate the effect of combined treatment on the tumor microenvironment. |
| Final Deliverables | Estimated Timeframe |
| Clients will receive detailed study reports with comprehensive data analysis, optimized oncolytic virus constructs, and in vitro and in vivo experimental results presented clearly and concisely. | The typical timeframe for this service ranges from 12 to 20 weeks, depending on the complexity of the oncolytic virus design, the specific requirements of the in vivo models, and the scope of the study. |
[Discover How We Can Help - Request a Consultation]
Case Study
The implementation of combination therapy combining genetically engineered oncolytic viruses and immune checkpoint inhibitors in commonly employed in vivo rodent models and in vitro tumor cell line models, including those for ovarian cancer, has resulted in a significant enhancement of anti-tumor efficacy. Findings from numerous published research studies provide valuable perspectives on its considerable potential for treating a wide range of cancers, particularly ovarian cancer.
| Cytotoxicity | Tumor Growth Monitoring |
|---|---|
|
|
|
| Survival Curve | Animal Weight Monitoring |
|
|
|
| Flow Cytometry | Elisa |
|
|
|
| Western Blot | PD-1/PD-L1 Binding Assay |
|
|
|
Customer Reviews
-
"Using Creative Biolabs' oncolytic viruses in our research has significantly improved the efficacy of our ICI therapy."
J**n Smith[3 Months]
-
" Creative Biolabs' expertise in virus engineering facilitated the rapid development of a novel oncolytic virus that enhances ICI activity."
M**k Brown[6 Months]
-
"The high quality of Creative Biolabs' GMP-grade oncolytic virus preparations was crucial for our clinical trial success. Using CB's Oncolytic Virotherapy has significantly improved our chances of success."
S**a Lee[12 Months]
[Experience the CB Advantage-Get a Quote Today]
What We Can Offer
As authorities in oncolytic virotherapy development for combination treatment with immune checkpoint inhibitors, Creative Biolabs presents an extensive range of services aimed at expediting your research and development initiatives. We comprehend the intricacies of immuno-oncology and are dedicated to furnishing you with the resources and proficiency required for achievement. Here's what we bring to the table:
- Customized Oncolytic Virus Design: We don't offer a one-size-fits-all solution. Creative Biolabs tailors the design of oncolytic viruses to your specific needs, including the expression of relevant transgenes (e.g., cytokines, checkpoint inhibitors) to maximize synergy with your ICI of choice.
- Comprehensive Preclinical Validation: From in vitro assays to complex in vivo models, we rigorously evaluate the efficacy and safety of our oncolytic virus constructs in combination with ICIs. This includes a detailed analysis of tumor response, immune cell activation, and biomarker expression.
- GMP-Grade Virus Production: Creative Biolabs provides scalable, GMP-compliant production of oncolytic viruses, ensuring a seamless transition from preclinical studies to clinical trials.
- Regulatory Support: Navigating the regulatory landscape can be daunting. We offer expert guidance and documentation support to help you meet the requirements for IND submission and clinical trial authorization.
- Deep Expertise: Our team comprises leading scientists with extensive experience in virology, immunology, and oncology. We stay at the forefront of the field, bringing the latest advances to your projects.
- Collaboration and Partnership: We view our clients as partners. Creative Biolabs works closely with you throughout the entire process, providing ongoing support, communication, and collaboration to ensure your project's success.
Introduction of Oncolytic Virotherapy
Oncolytic viruses (OVs) are a promising class of immunotherapeutic agents that selectively replicate in and lyse tumor cells while sparing normal tissues. This process not only directly destroys cancer cells but also stimulates a potent anti-tumor immune response. The development of oncolytic virotherapy has focused on enhancing virus selectivity, potency, and safety through genetic engineering. Preclinical and clinical studies have demonstrated the potential of OVs as a monotherapy and, importantly, in combination with other immunotherapies like ICIs.
Oncolytic Virotherapy Development for Combination Therapy with Immune Checkpoint Inhibitors
Combining oncolytic virotherapy with immune checkpoint inhibitors (ICIs) is a powerful, synergistic cancer immunotherapy approach. Oncolytic viruses reshape the tumor microenvironment, boosting immune cell activity and enhancing ICI efficacy. ICIs work by unblocking proteins that suppress T-cell anti-cancer attacks, but many tumors develop resistance to them. This makes the oncolytic virotherapy-ICI combination highly promising, as it could overcome these obstacles and improve treatment outcomes. However, many tumors develop resistance to ICIs.
Tab.1 List of ongoing combination therapy clinical trials with oncolytic viruses and immune checkpoint inhibitors.4,5
| Oncolytic Virus | Variant | Modification | Combination | Tumor types |
|---|---|---|---|---|
| Adenovirus | CG007 | GM-CSF | Anti-PD-1 antibody | Bladder cancer |
| Ads/HSV-tk | HSV-tk | Anti-PD-1 antibody | TNBC, NCLC | |
| Enadenotucirev | A11/Ad3 Chimeric Backbone | Anti-PD-1 antibody | Advanced epithelial tumors | |
| Telomelysin | hTERT | Anti-PD-1 antibody | Advanced solid tumors | |
| HSV-1 | HF-10 | Spontaneously mutated | Anti-CTLA-4 antibody | Melanoma |
| Imlygic | GM-CSF | Anti-PD-1 antibody | HNSCC | |
| Vaccine Virus | Pexa-Vac | Human GM-CSF+β-galactosidase | Anti-CTLA-4 antibody | Advanced solid tumors |
| Anti-PD-1 antibody | HCC | |||
| Coxsackievirus | CAVATEK | Non | Anti-CTLA-4 antibody | Melanoma |
| VSV | VSV- IFNβ-NIS | IFNβ and sodium importer gene | Anti-PD-1 antibody | NSCLC and HCC |
Creative Biolabs offers a comprehensive suite of services to support the development of oncolytic virotherapy for combination therapy with ICIs, from virus design and engineering to preclinical and clinical study support. If you have any need, please feel free to contact us!
References
- Tesfay, Mulu Z., et al. "Enhancing immune response and survival in hepatocellular carcinoma with novel oncolytic Jurona virus and immune checkpoint blockade." Molecular Therapy Oncology 32.4 (2024). DOI: 10.1016/j.omton.2024.200913.
- Heiniö, Camilla, et al. "Effective combination immunotherapy with oncolytic adenovirus and anti-PD-1 for treatment of human and murine ovarian cancers." Diseases 10.3 (2022): 52. DOI: 10.3390/diseases10030052.
- Klawitter, Moritz, et al. "The oncolytic adenovirus XVir-N-31, in combination with the blockade of the PD-1/PD-L1 axis, conveys abscopal effects in a humanized glioblastoma mouse model." International Journal of Molecular Sciences 23.17 (2022): 9965. DOI: 10.3390/ijms23179965.
- Hwang, June Kyu, JinWoo Hong, and Chae-Ok Yun. "Oncolytic viruses and immune checkpoint inhibitors: preclinical developments to clinical trials." International journal of molecular sciences 21.22 (2020): 8627. DOI: 10.3390/ijms21228627.
- Distributed under Open Access license CC BY 4.0, Some of the pictures were edited and reformatted.

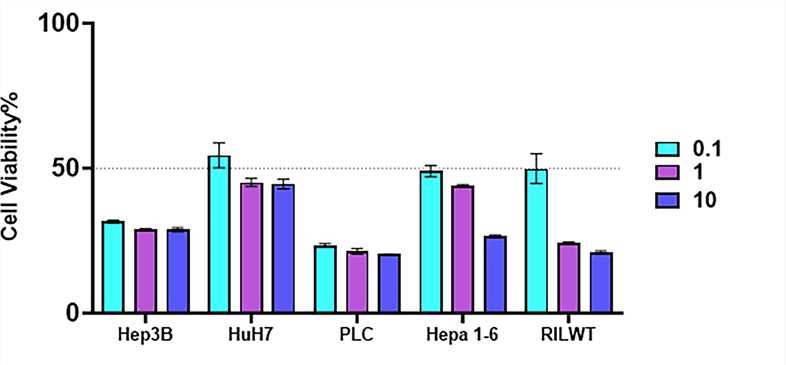 Fig.1 The percentage of cell viability is determined using a colorimetric assay.1,5
Fig.1 The percentage of cell viability is determined using a colorimetric assay.1,5
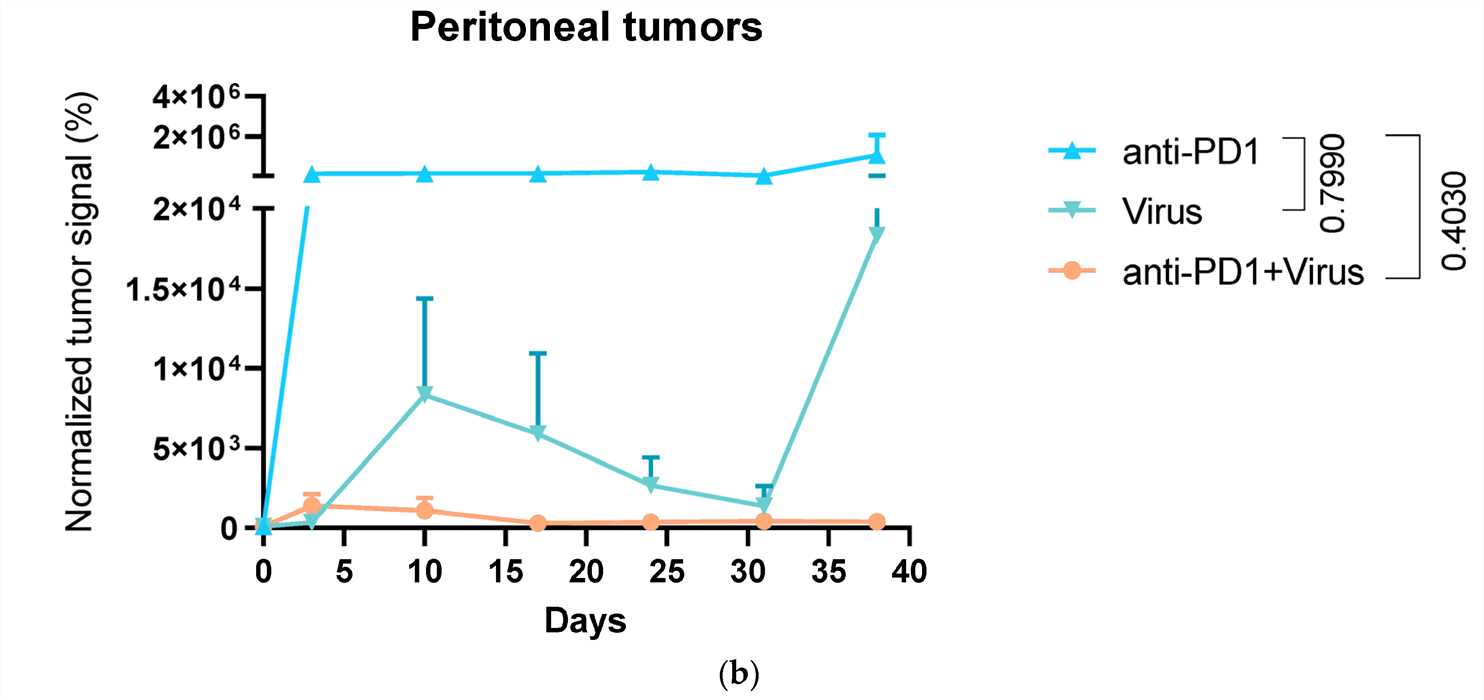 Fig.2 Fluorescence real-time imaging is used to monitor tumor size.2,5
Fig.2 Fluorescence real-time imaging is used to monitor tumor size.2,5
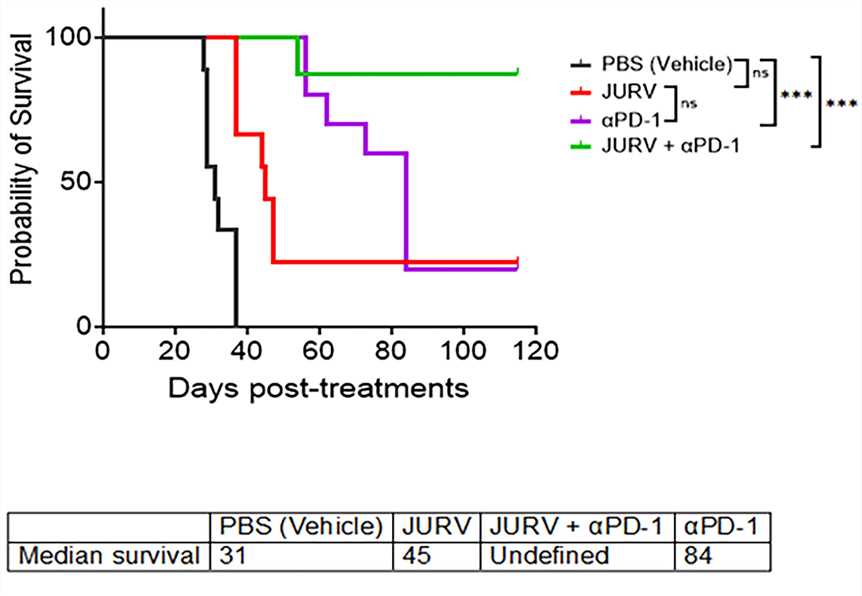 Fig.3 Kaplan-Meier survival curves.1,5
Fig.3 Kaplan-Meier survival curves.1,5
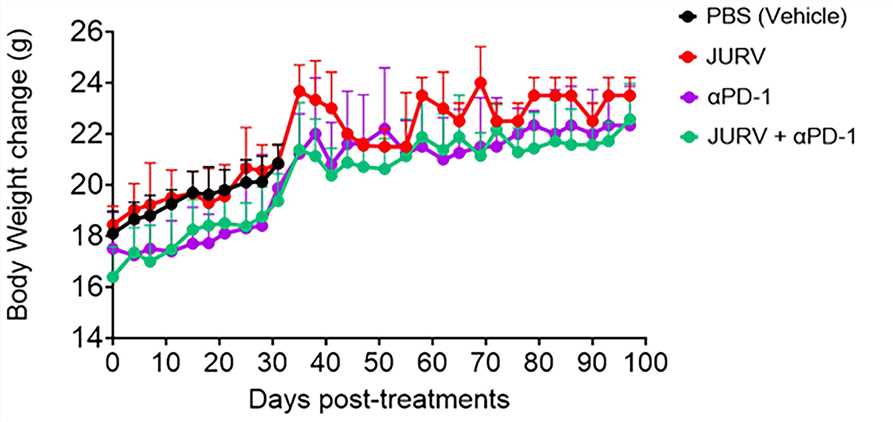 Fig.4 Changes in body weight of mice over time after treatment.1,5
Fig.4 Changes in body weight of mice over time after treatment.1,5
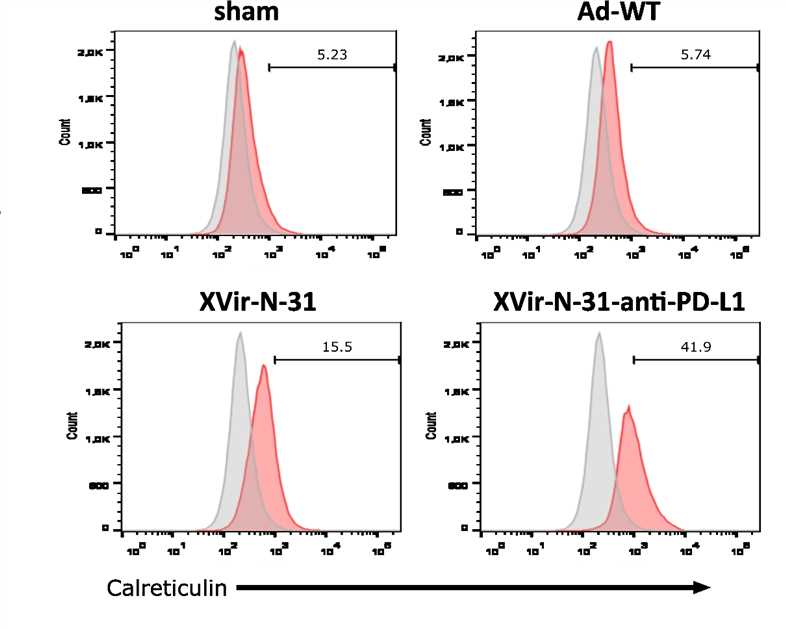 Fig.5 Flow cytometry is used to detect the death of tumor cells.3,5
Fig.5 Flow cytometry is used to detect the death of tumor cells.3,5
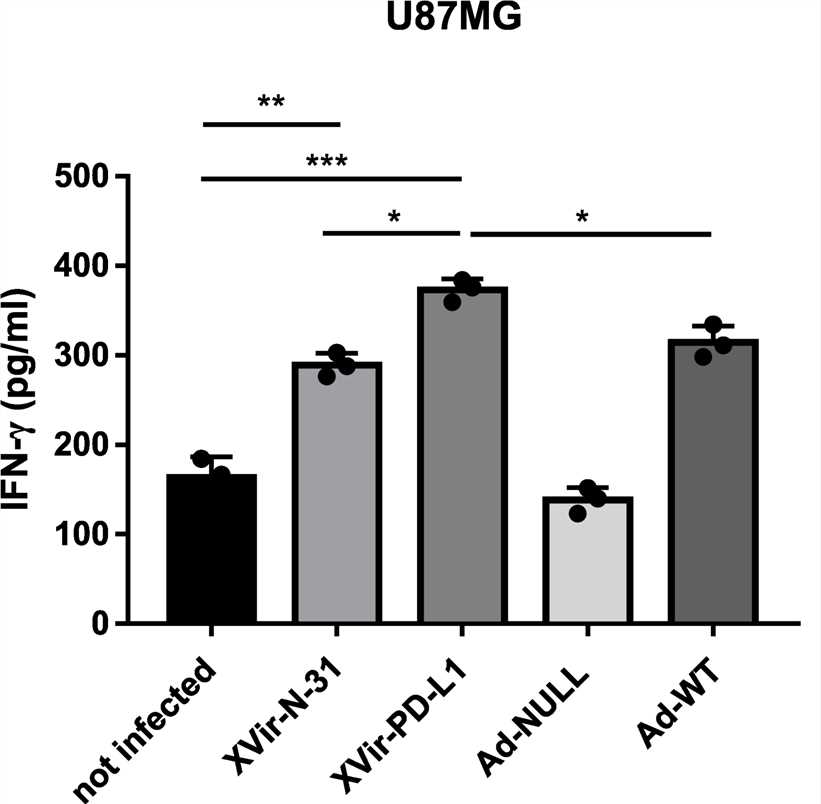 Fig.6 Eilsa assay was used to detect the secretion of target cytokines.3,5
Fig.6 Eilsa assay was used to detect the secretion of target cytokines.3,5
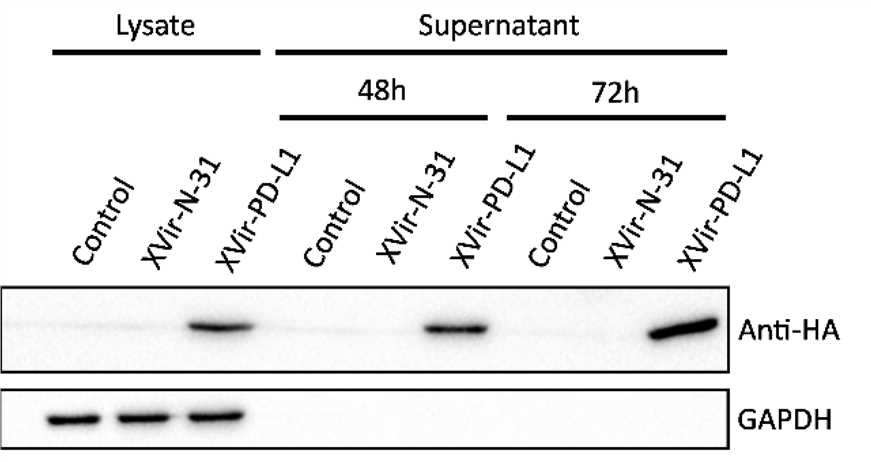 Fig.7 Western blot is used to analyze the expression of target proteins.3,5
Fig.7 Western blot is used to analyze the expression of target proteins.3,5
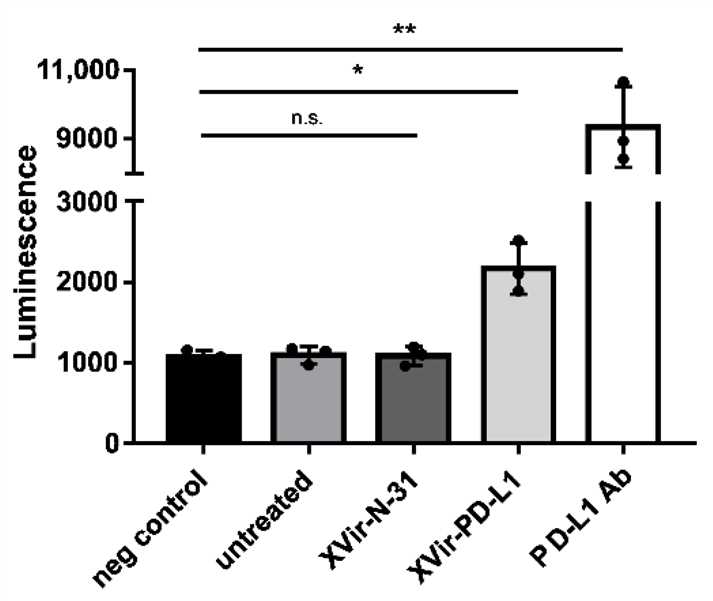 Fig.8 PD-1/PD-L1 Binding Assay.3,5
Fig.8 PD-1/PD-L1 Binding Assay.3,5