Breast Cancer Specific Oncolytic Virotherapy Development Service
Introduction
Breast cancer is a malignancy that typically arises in the breast tissue, often initiated in the cells of the milk ducts or lobules. Neoplastic cells in the breast of affected patients undergo unregulated proliferation. These cells can infiltrate adjacent tissues and have the potential to metastasize to distant body sites. Amid the swift advancement of innovative therapeutic approaches in breast cancer, oncolytic viruses (OVs) persist in showing great potential as an efficacious treatment option. Leveraging our OncoVirapy™ platform, Creative Biolabs is assured of offering novel oncolytic viruses and formulating more effective oncolytic virotherapy strategies for our client.s
Factors Predisposing to Breast Cancer
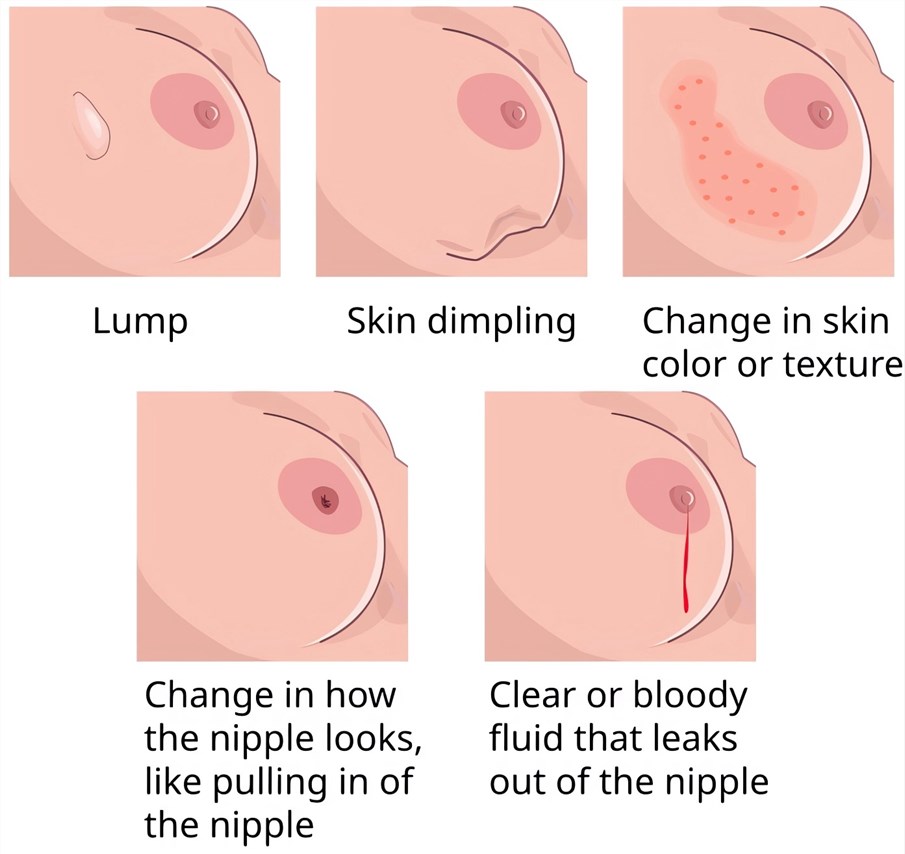 Distributed under CC BY-SA 4.0, from Wiki, without modification.
Distributed under CC BY-SA 4.0, from Wiki, without modification.
Breast cancer represents a malignant transformation of breast cells, predominantly originating from the mammary glands or ducts within the breast. The risk of developing breast cancer is elevated in women who carry the breast-cancer-susceptibility genes BRCA1 or BRCA2, those who are over 50 years old, individuals with a personal history of breast cancer, and women with a family history of breast cancer among their mothers, sisters, or daughters. However, it's important to note that women lacking these risk factors can still be affected by the disease. Typically, the initial symptom manifests as a painless mass in the breast. As the cancer progresses, the mass enlarges, becomes firm, and is immobile. Additionally, the overlying skin may exhibit warmth, erythema, and swelling, presenting an appearance similar to that of an orange peel. In cases where breast cancer has metastasized, symptoms may first emerge in other parts of the body, such as dyspnea, bone pain, or signs of osteopenia.
In addition to common drug delivery and low efficacy issues, breast cancer treatment has unique challenges. These factors involve characteristics specific to the patient, such as variations in the female immune system and the influence of menopause on immune function. Additionally, there are characteristics specific to the tumor, including the estrogen receptor (ER) and human epidermal growth factor receptor 2 (HER2) status, as well as the application of anti-estrogen therapies.
Breast cancer mainly affects estrogen-dependent women with upregulated estrogen receptors. Combining anti-estrogen therapy and breast cancer checkpoint inhibition shows potential. However, men and women respond differently to checkpoint inhibition, perhaps because estrogen, acting as a steroid hormone in the immune system, modulates the tumor environment and adaptive immunity.
Oncolytic Virus Therapy for Breast Cancer
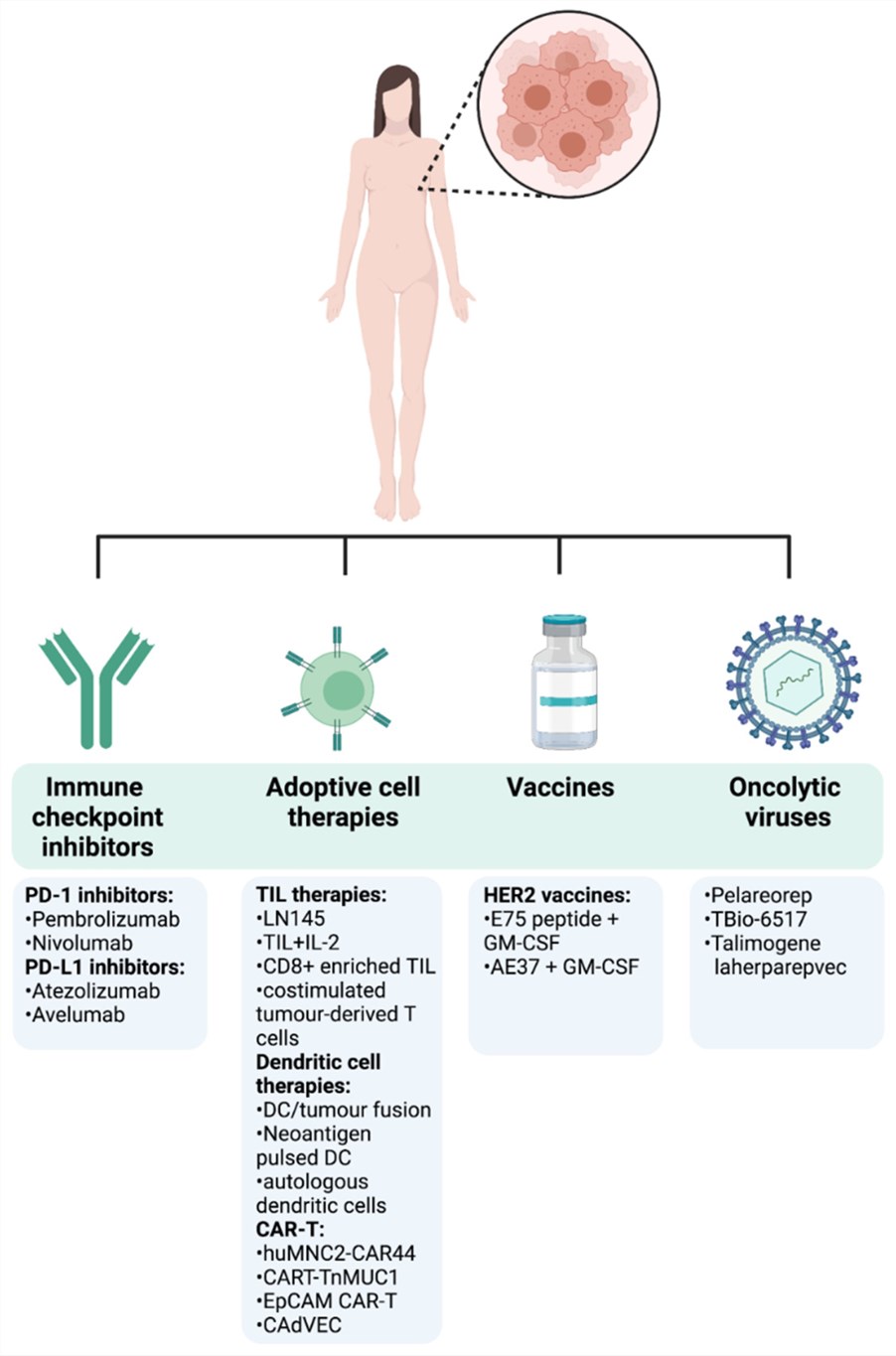 Fig.1 Immunotherapy approaches in breast cancer.1
Fig.1 Immunotherapy approaches in breast cancer.1
- HSV
Clinical trials are assessing T-VEC's safety and efficacy in TNBC patients. G47Δ-mIL12 shows anti-tumor activity in the 4T1 breast cancer model, acting on TNBC cells in vitro and inhibiting tumor growth/metastasis in vivo. HF10, a mutated HSV-1, elicits anti-tumor immunity in colon/breast cancer mouse models, and its phase I trial indicates immune response induction.
- Adenovirus
ONYX-015, an adenovirus with a deletion in the E1B region that knocks out p53, is currently being tested in a phase Ⅰ trial when used together with etanercept. Besides, a new recombinant adenovirus that zeroes in on E2F-1 and has the IL15 gene added to it can stop breast cancer cells from multiplying. The fact that the transcription factor E2F-1 is highly expressed in breast cancer tissues suggests that E2F-1 can be a really good target for treatment. What's more, by carrying the IL15 gene, this oncolytic adenovirus can trigger the creation and activation of effector T cells and control how memory T cells survive and multiply, giving it the ability to adjust the immune response.
- NDV
In vitro and in vivo data indicate that diverse strains of oncolytic Newcastle disease virus (NDV) hold promise as novel approaches for breast cancer treatment. A newly developed recombinant NDV expressing rAF-IL12 is found to significantly impede tumor growth in treated mice. More recently, it has been demonstrated that the combination of NDV virotherapy and the glucose analog 2-DG potentiates antitumor effects in both human and mouse breast cancer cells. These findings offer the potential for the application of OVs, particularly NDVs, in various strategies for breast cancer treatment.
Workflow
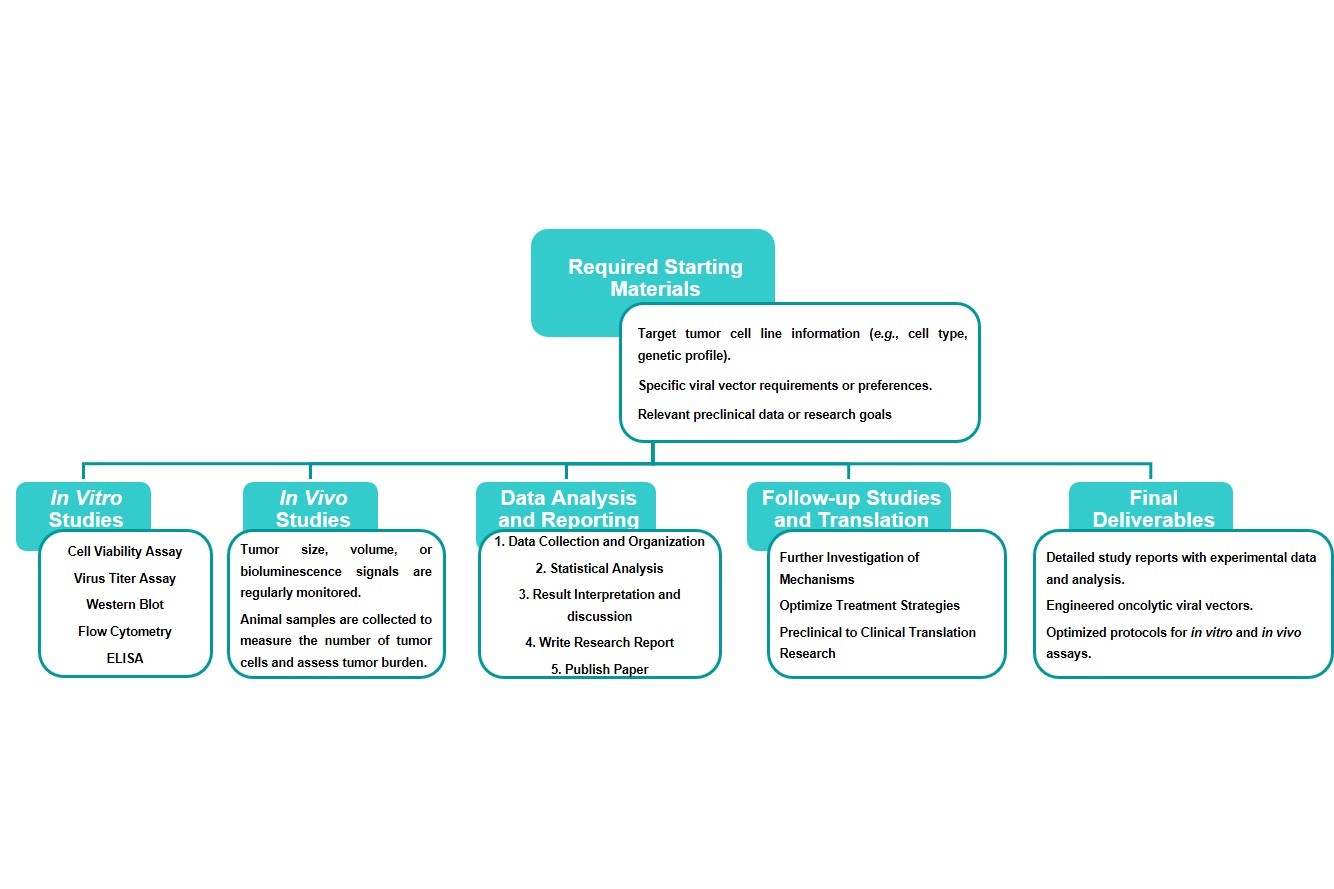
Estimated Timeframe:
Pre-requirement communication: 1-2 weeks
Design and construction of oncolytic viruses: 3-4 weeks
Mass production of oncolytic viruses: 2-3 weeks
Function and properties of oncolytic viruses in vivo and in vitro: 3-4 weeks
Results analysis and test report: 1-2 weeks
Product delivery and shipping: 2-3 weeks
Case Study
The deployment of genetically - engineered oncolytic viruses in commonly utilized in vivo murine models and in vitro tumor cell line models of breast cancer has demonstrated a significant elevation in tumor dissolution rates. Data aggregated from a series of published academic articles provide meaningful perspectives on its promising prospects for breast cancer treatment.
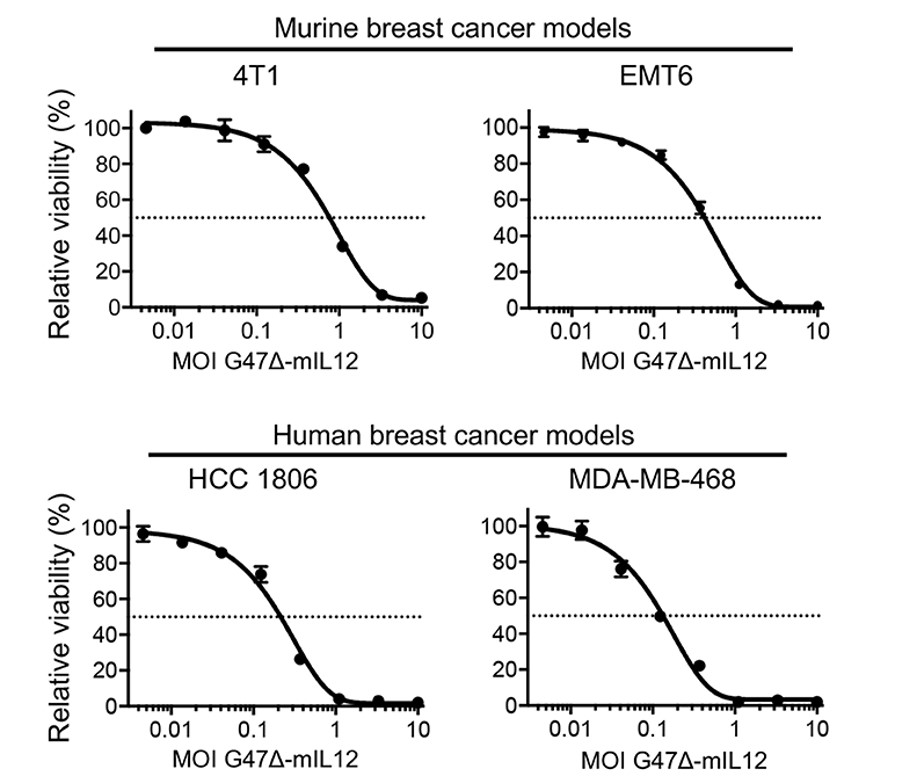 Fig.2 Oncolytic viruses effectively reduce the viability of human and murine breast cancer cells.2,5
Fig.2 Oncolytic viruses effectively reduce the viability of human and murine breast cancer cells.2,5
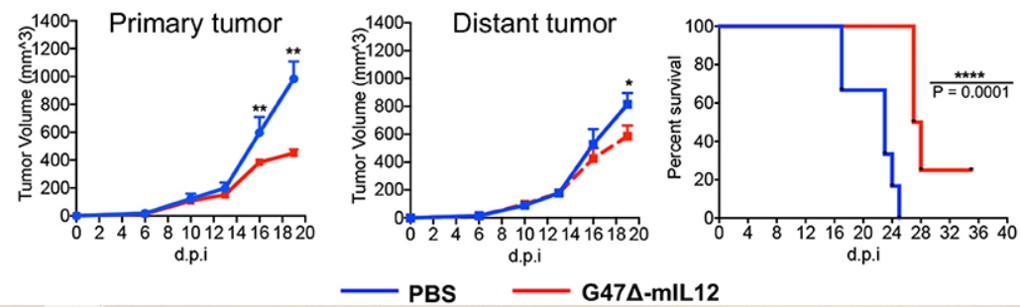 Fig.3 Oncolytic virus can reduce the volume of breast tumors and prolong the survival time of tumor-bearing mice.2,5
Fig.3 Oncolytic virus can reduce the volume of breast tumors and prolong the survival time of tumor-bearing mice.2,5
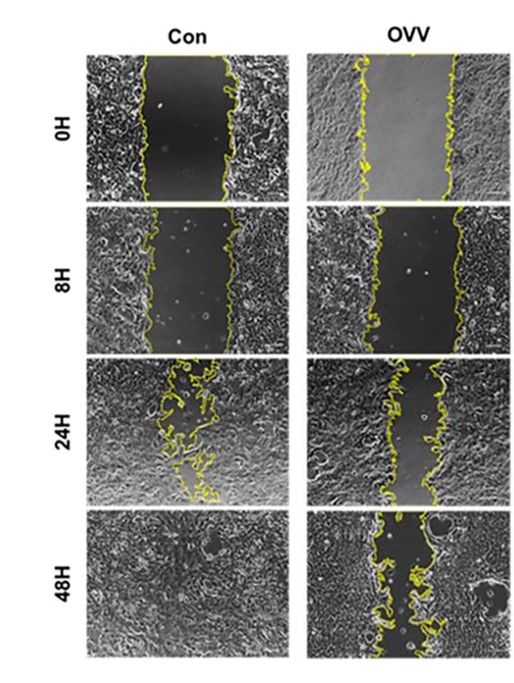 Fig.4 Migration assay is used to determine the effect of oncolytic virus on breast cancer cell death induction.3,5
Fig.4 Migration assay is used to determine the effect of oncolytic virus on breast cancer cell death induction.3,5
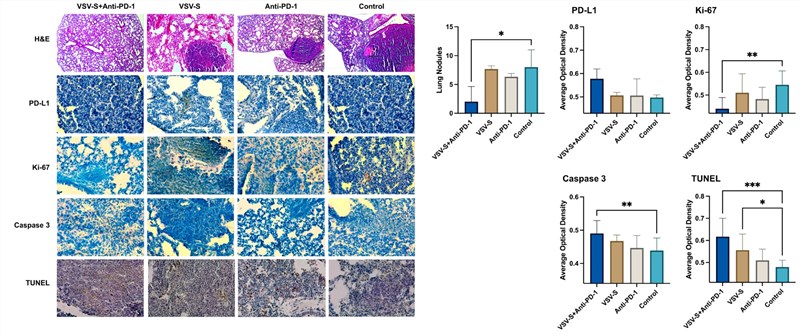 Fig.5 Immunohistochemical staining is used to determine the expression of target markers.4,5
Fig.5 Immunohistochemical staining is used to determine the expression of target markers.4,5
References
- Cilibrasi, Chiara, et al. "Reconstituting immune surveillance in breast cancer: Molecular pathophysiology and current immunotherapy strategies." International journal of molecular sciences 22.21 (2021): 12015. DOI: 10.3390/ijms222112015. Distributed under Open Access license CC BY 4.0, without modification.
- Ghouse, Shanawaz M., et al. "Oncolytic herpes simplex virus encoding IL12 controls triple-negative breast cancer growth and metastasis." Frontiers in oncology 10 (2020): 384. DOI: 10.3389/fonc.2020.00384
- Kim, Hyo-Sung, et al. "Enhanced Antitumor Efficacy of Oncolytic Vaccinia Virus Therapy Through Keratin-Mediated Delivery in Triple-Negative Breast Cancer." International Journal of Molecular Sciences 25.21 (2024): 11470. DOI: 10.3390/ijms252111470
- Tang, Sijia, et al. "Enhancing the Efficacy of Breast Cancer Immunotherapy Using a Smac-Armed Oncolytic Virus." Cancers 16.19 (2024): 3248. DOI: 10.3390/cancers16193248
- Distributed under Open Access license CC BY 4.0, reformat the picture.
