Head & Neck Cancer (HNC) Specific Oncolytic Virotherapy Development Service
HNC stands as the world's sixth most prevalent cancer type which makes up 6% of all cancer cases and results in 350,000 deaths annually. HNC includes cervical tumors, otolaryngological (ENT) tumors, and oral and maxillofacial tumors. Among cervical tumors, thyroid tumors are the most prevalent. In otolaryngology, common tumors comprise laryngeal cancer, paranasal sinus cancer, and so on. Tongue cancer, gingival cancer, and buccal cancer represent frequent forms of oral cancer. The majority of head and neck cancer occurrences represent squamous cell carcinomas which develop primarily in areas like the oropharynx, oral cavity, hypopharynx, or larynx.
The treatment options for head and neck cancer encompass surgery, radiotherapy, chemotherapy, targeted therapy, and immunotherapy. Oncolytic virotherapy for HNC represents a relatively advanced therapeutic approach currently. Through gene editing techniques, the oncolytic viruses (Ovs) can be engineered to reduce their virulence to normal cells. Administration routes include intratumoral injection and intravenous infusion, both of which exhibit high safety profiles.
Staffed with preeminent experts and armed with rich experience in immunotherapy, Creative Biolabs has set up a cutting-edge OncoVirapy™ platform, through which its scientists are adept at devising tailored oncolytic virotherapy plans for head and neck cancer.
Factors Predisposing to Lung Cancer
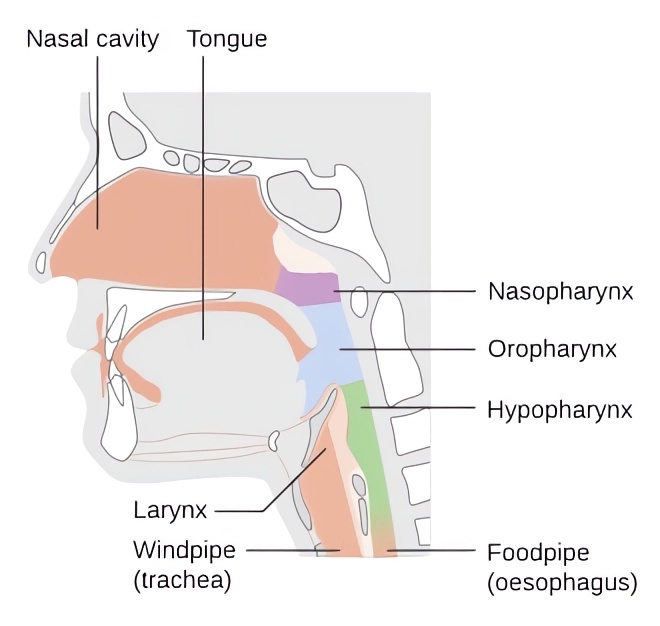 Fig.1 Schematic diagram of the anatomy of the throat.Distributed under CC BY-SA 4.0, from Wiki, without modification.
Fig.1 Schematic diagram of the anatomy of the throat.Distributed under CC BY-SA 4.0, from Wiki, without modification.
Tab.1 The basic components of head and neck cancer, pathogenic factors, and treatment.
| Head and Neck Cancer | Detailed information |
|---|---|
| Oral Cancer |
|
| Pharyngeal Cancer |
|
| Laryngeal Cancer |
|
| Thyroid Cancer |
|
| Tongue cancer | Most of them occur at the lingual margin, followed by the tip of the tongue, the back of the tongue, and the base of the tongue. Most of the pathological types were squamous cell carcinoma |
| Pathogenic factors |
|
| Main treatment |
|
Oncolytic Virus Therapy for Head and Neck Cancer
The application of oncolytic virus therapy represents a promising approach to treating head and neck cancer. OVs show significant promise as a treatment option for head and neck cancer. Human gene therapy applications in HNC research utilize viruses such as lentiviruses, adenoviruses, herpes simplex viruses, vaccinia virus, and adeno-associated viruses which possess specific properties enabling selective targeting and destruction of cancer cells with minimal damage to healthy tissue.
- EGFR is highly overexpressed in numerous HNSCCs. Human Ad5 modified with the EGFR-binding protein ligand can precisely target the overexpressed EGFR in HNSCC.
- RAd-p53, a gene therapy agent, is approved for HNSCC patients with TP53 mutations. It is generated by cotransformation-relevant vectors in HEK 293 cells. In HNC patients, its overall response rate (CR+PR) exceeds 90%, much higher than standard cancer therapy alone. Most regimens combine it with radiotherapy and/or chemotherapy.
- G47Δ is a triple-genetically-engineered, third-generation oncolytic HSV-1 that has been genetically modified to lose γ34.5 and α47 and inactivate the ICP6. In patients with untreated oral squamous cell carcinoma, adjunctive use of G47Δ with a monoclonal antibody inhibits tumor growth in the pre-surgical period and also eliminates residual microtumors in the residual tongue.
- HF10 is likewise a highly attenuated, replication-competent mutant oHSV-1. It has obvious toxicity to tongue cancer cells in vitro, strong replication ability, and good tumor cell lysis ability by intratumoral injection.
Workflow
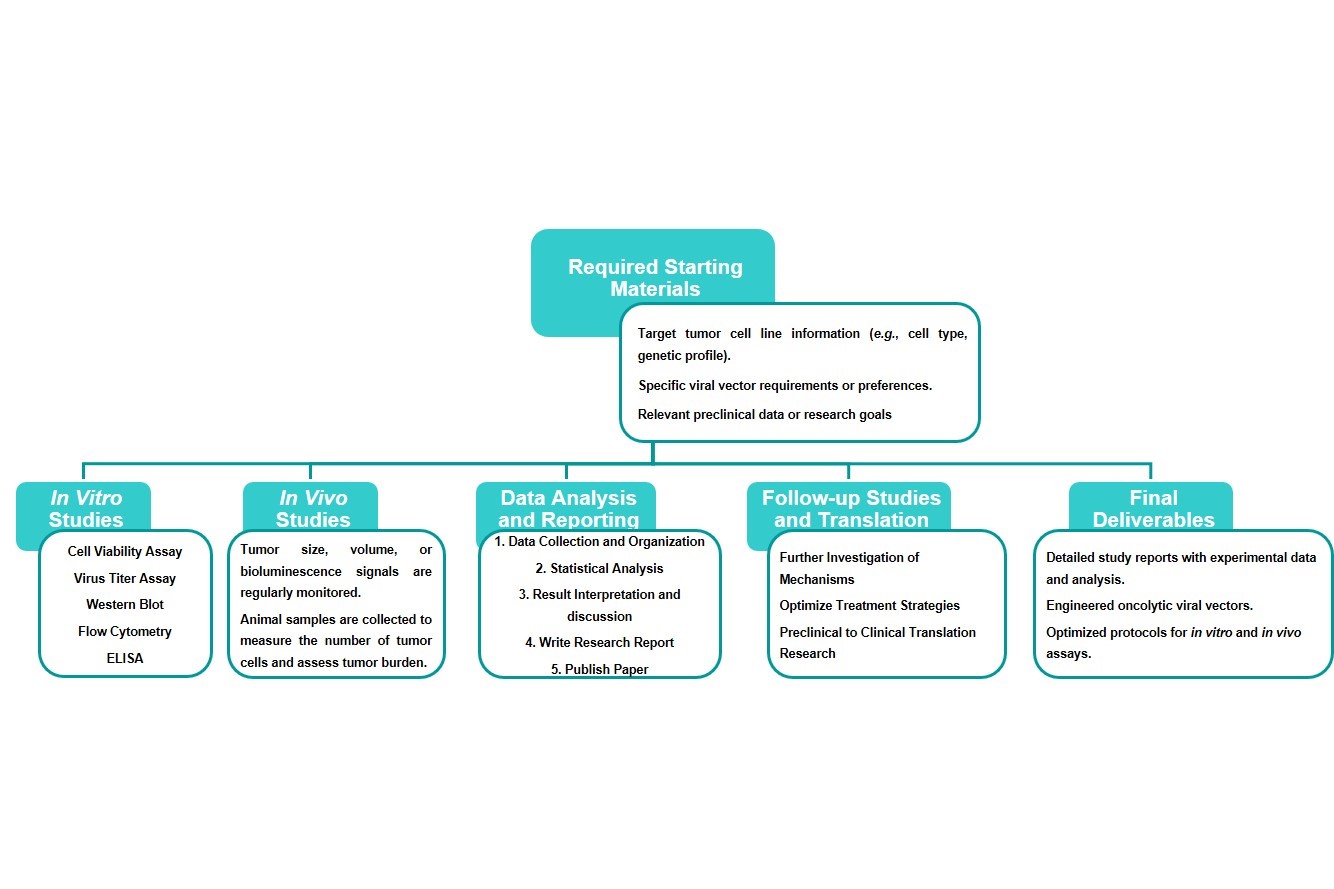
Estimated Timeframe:
Pre-requirement communication:1-2 weeks
Design and construction of oncolytic viruses: 3-4 weeks
Mass production of oncolytic viruses:2-3 weeks
Function and properties of oncolytic viruses in vivo and in vitro:3-4 weeks
Results analysis and test report:1-2 weeks
Product delivery and shipping:2-3 weeks
Case Study
The use of genetically engineered oncolytic viruses in commonly employed in vivo mouse models and in vitro cell-culture models of HNC has shown a significant rise in the rates of tumor destruction. Information gathered from several published research data provides useful evidence on OVs promising potential for treating HNC.
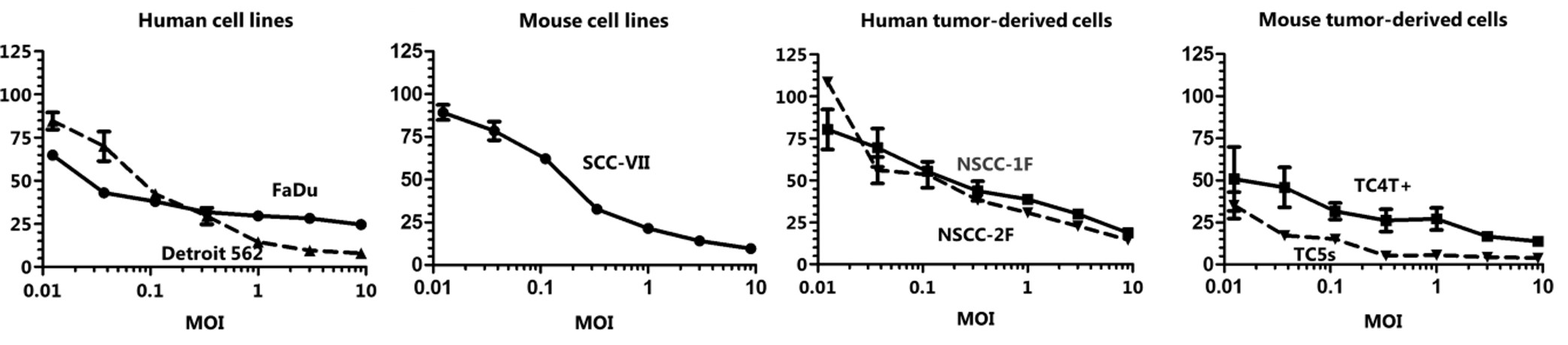 Fig.2 Oncolytic HSV reduces the activity of HNC cells of different origins.1
Fig.2 Oncolytic HSV reduces the activity of HNC cells of different origins.1
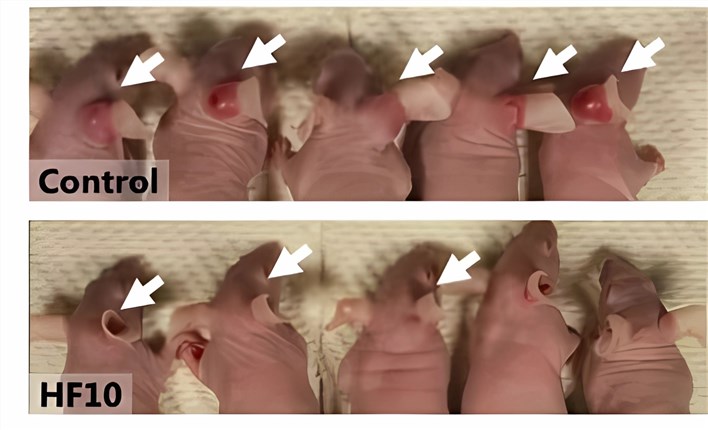 Fig.3 Oncolytic HSV is effective in eliminating tumors.1
Fig.3 Oncolytic HSV is effective in eliminating tumors.1
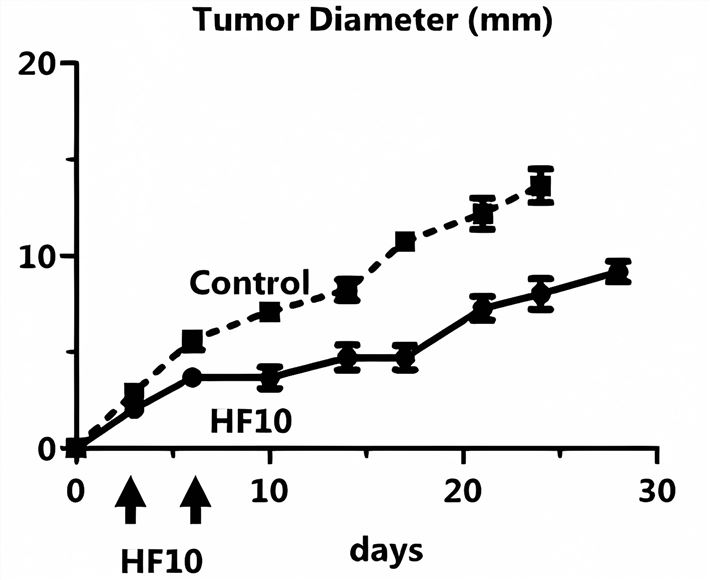 Fig.4 Oncolytic HSV is effective in eliminating tumors.1
Fig.4 Oncolytic HSV is effective in eliminating tumors.1
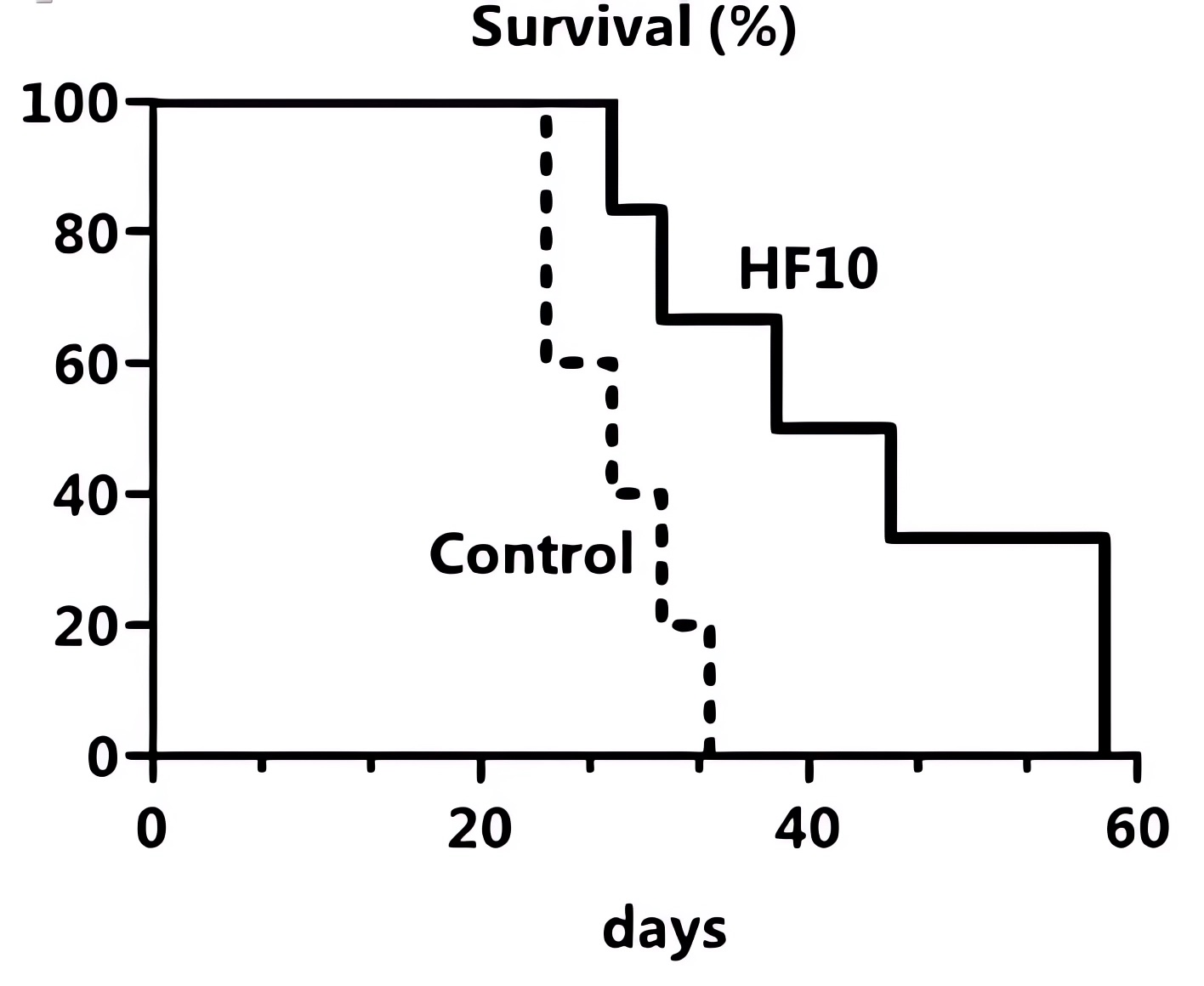 Fig.5 Oncolytic HSV treatment can significantly prolong the survival time of HNC model mice.1
Fig.5 Oncolytic HSV treatment can significantly prolong the survival time of HNC model mice.1
FAQ
- How to accurately assess the oncolytic potency of oncolytic viruses?
Creative Biolabs provides a variety of experimental protocols to accurately assess the efficacy of oncolytic virus products. For example, it uses MTT assay or CCK-8 assay, flow cytometry, western blot, etc. With the mature oncolytic virus construction and detection platform, Creative Biolabs' experienced technical team will offer customers professional customized in vivo and in vitro experimental protocol design.
- How to verify the effect in vivo and ensure the success rate of animal model construction?
Animal models of HNC are mainly constructed using immunodeficient or transgenic mice. Human HNC cell lines or tumor tissues are transplanted to form subcutaneous or orthotopic models. Modeling success is enhanced by optimizing cell inoculation (dose, site) and monitoring animal health or tumor growth. Advanced imaging, like in vivo imaging systems, enables real-time tumor observation to meet experimental needs.
- What additional information is needed to design and construct oncolytic viruses?
First, we identify the oncolytic virus types clients need, such as adenovirus, herpes simplex virus, and vaccinia virus. Clients should provide accurate gene sequences, functions, and expression regulations if there are genes for insertion or deletion. Also, clients need to define molecular targets and functional detection indicators. Our professionals will communicate with clients to craft the plan.
- In addition to animal and cell models, are there other means to test oncolytic virus function?
In addition to in vitro cell models and animal models, the functionality of oncolytic viruses can also be evaluated by constructing relevant tumor organoids. The construction duration typically ranges from 4 to 6 weeks. Throughout this period, our team will maintain communication with you and provide regular feedback on the growth status of the organoids.
In the face of the swift advancements in innovative therapeutic approaches for HNC, oncolytic viruses remain a highly promising treatment modality. Leveraging our state-of-the-art OncoVirapy™ platform, Creative Biolabs is certain of its ability to supply novel OVs and formulate more effective oncolytic virotherapy strategies for our clients. Should you have any inquiries regarding oncolytic viruses, please do not hesitate to reach out to us.
Reference
- Esaki, Shinichi, et al. "Oncolytic activity of HF10 in head and neck squamous cell carcinomas." Cancer Gene Therapy 27.7 (2020): 585-598. DOI: 10.1038/s41417-019-0129-3. Distributed under Open Access license CC BY 4.0, the figures are reformatted.
