Sarcoma Specific Oncolytic Virotherapy Development Service
Accelerate Your Sarcoma Research with Cutting-Edge Oncolytic Virotherapy Solutions!
Are you struggling with challenges in developing effective treatments for sarcomas, such as the limited success in handling metastatic and relapsed cases, along with the complexity of targeting this varied group of tumors?
Our Creative Biolabs Sarcoma Oncolytic Virotherapy Development service allows you to accelerate the research and creation of novel oncolytic virus-based treatments. We utilize state-of-the-art oncolytic virus engineering and an all-inclusive OncoVirapy™ platform to offer you customized solutions.
How Creative Biolabs Assists Your Project
- Custom-designed oncolytic viruses tailored to specific sarcoma types.
- Comprehensive support throughout the entire development process, from virus engineering to validation.
- Access to cutting-edge technology and expertise in oncolytic virus engineering.
- Strategies to enhance the safety and efficacy of oncolytic virus therapies.
[Discover How We Can Help - Request a Consultation]
Workflow
Estimated Timeframe:
Pre-requirement communication: 1-2 weeks
Design and construction of oncolytic viruses: 3-4 weeks
Mass production of oncolytic viruses: 2-3 weeks
Function and properties of oncolytic viruses in vivo and in vitro: 3-4 weeks
Results analysis and test report: 1-2 weeks
Product delivery and shipping: 2-3 weeks
What We Offer
Creative Biolabs offers a comprehensive suite of customized oncolytic virus development services for sarcoma research, including:
- Tailorable Oncolytic Virus Design: We design oncolytic viruses to fulfill your particular research objectives, such as augmenting tumor tropism and optimizing safety characteristics.
-
A Comprehensive OncoVirapy™ Platform:
- Documentation quality and procedures of strain origin are assessed and approved by our qualified quality assurance service.
- We guarantee the stability of strains in cell banks and large-scale fermentations, including pre-cultivation.
- Codon usage of genes is optimized to facilitate expression in selected microorganisms.
- The fermentation process can be run in batch, fed-batch, or continuous mode.
- Culture conditions are optimized to maximize yield.
- We take precautions and follow the Hazard Analysis Critical Control Point (HACCP) approach and the basic principles of Good Manufacturing Practice (GMP).
- High-standard quality control tools are used to quantify and evaluate the quality of products.
- Quality and Aseptic Production: We have a well-established quality system, incorporating Quality-by-Design (QbD) and process analytical techniques (PAT). We also employ strict aseptic verification procedures throughout the fermentation process and GMP-certified fermentation production.
- Commitment to Environmental Protection: We invest in environmental protection measures and skills.
Why Choose Us
- A comprehensive OncoVirapy™ platform with a wide range of oncolytic virus engineering capabilities.
- Expertise in a variety of oncolytic virus species, including adenovirus, herpes simplex virus, and vaccinia virus.
- Cutting-edge technologies for virus modification, including pathogenicity manipulation, immunogenicity manipulation, and transgene insertion.
- A dedicated team of experienced scientists and project managers committed to delivering high-quality results and exceptional customer service.
- Published Data supporting the efficacy of our oncolytic virus development services.
[Experience the CB Advantage-Get a Quote Today]
Case Study
The application of genetically engineered oncolytic viruses in frequently used in vivo murine models and in vitro tumor cell line models of sarcomas has resulted in a substantial increase in tumor cell lysis efficiency. Data extracted from several published research articles provide valuable perspectives on its favorable prospects for sarcomas therapy.
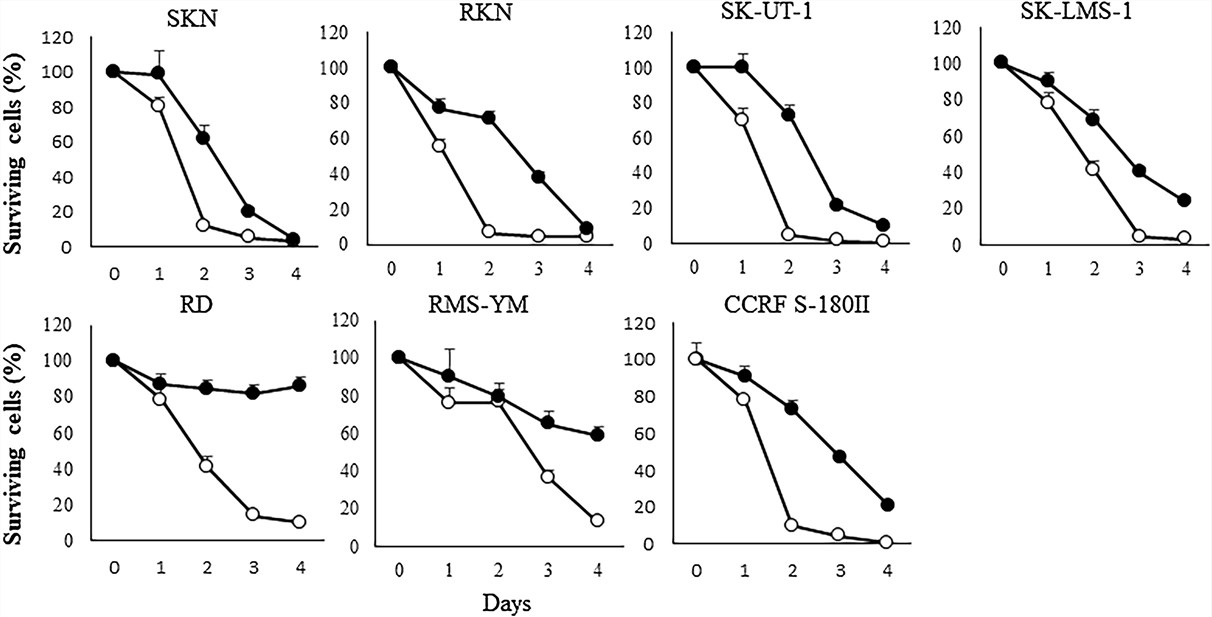 Fig.1 In vitro cytotoxicity assays for oncolytic viruses.1
Fig.1 In vitro cytotoxicity assays for oncolytic viruses.1
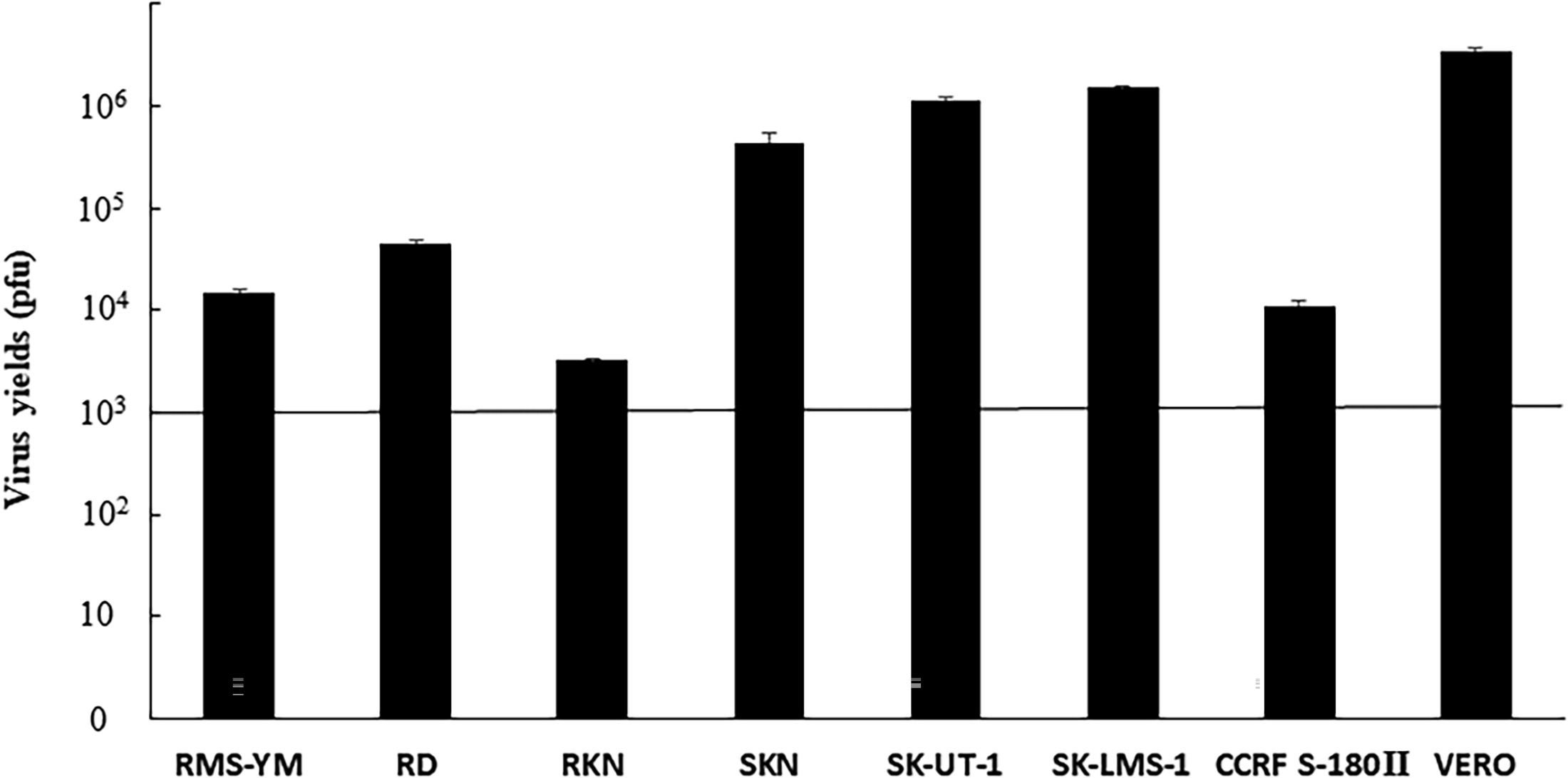 Fig.2 Detection of the replication capacity of oncolytic viruses.1
Fig.2 Detection of the replication capacity of oncolytic viruses.1
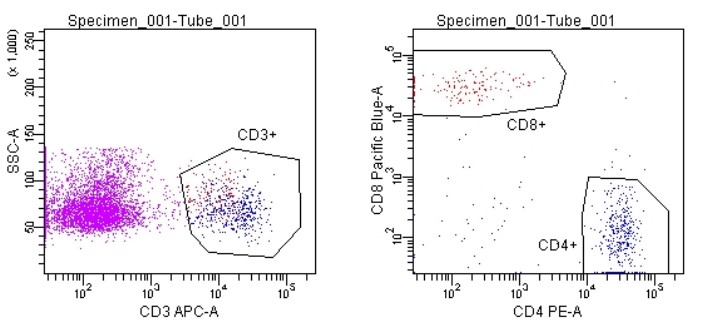 Fig.3 Flow cytometry was used to detect the changes in immune cells after oncolytic virus treatment.1
Fig.3 Flow cytometry was used to detect the changes in immune cells after oncolytic virus treatment.1
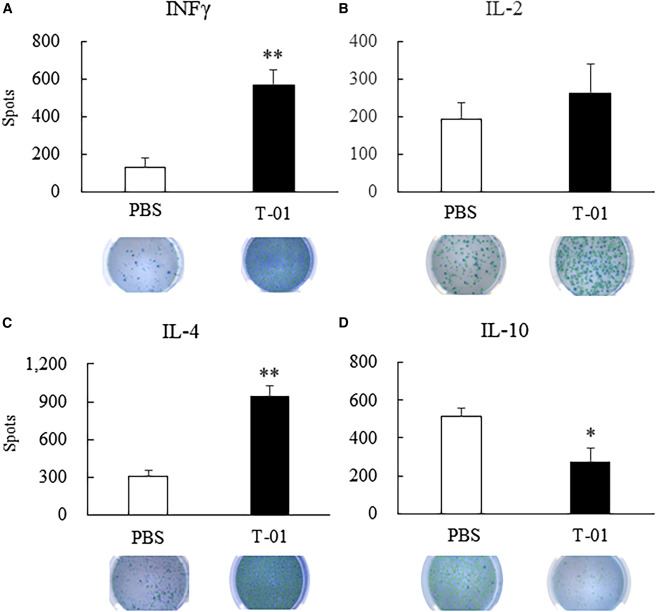 Fig.4 Detection of cytokine secretion of immune cells after oncolytic virus treatment.1
Fig.4 Detection of cytokine secretion of immune cells after oncolytic virus treatment.1
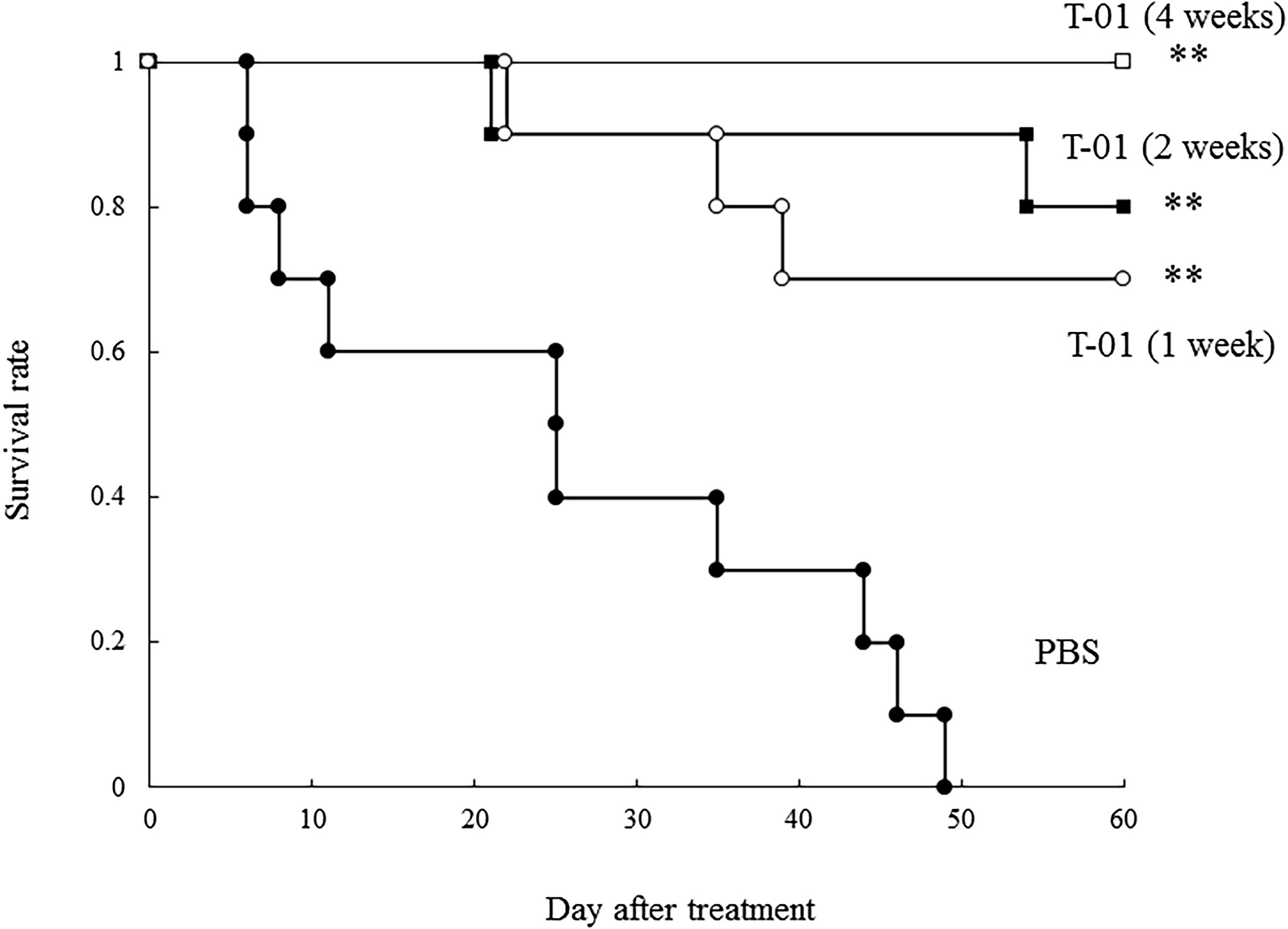 Fig.5 Oncolytic virus prolongs survival in cancer-bearing mice.1
Fig.5 Oncolytic virus prolongs survival in cancer-bearing mice.1
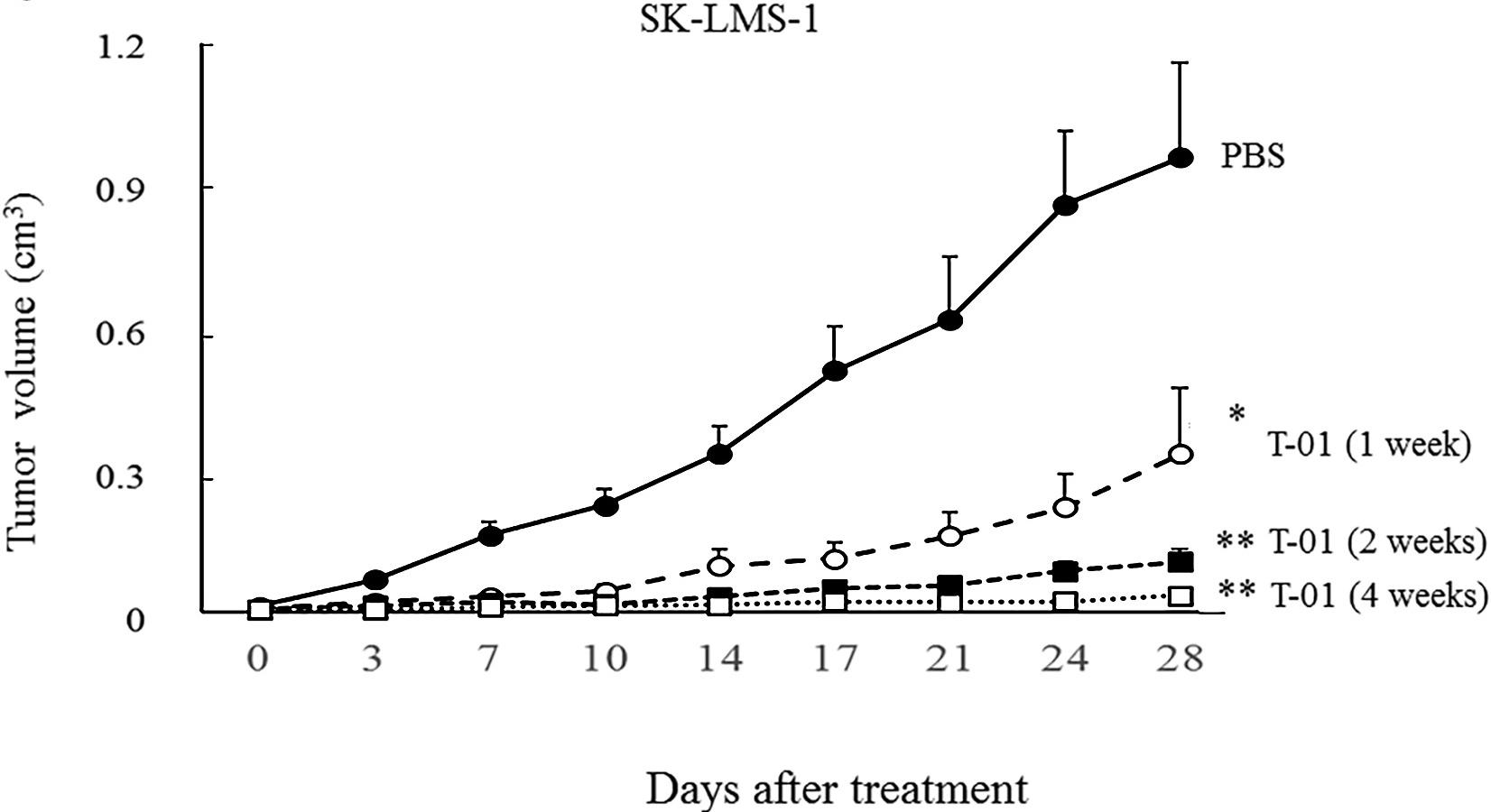 Fig.6 Oncolytic viruses inhibit tumor growth.1
Fig.6 Oncolytic viruses inhibit tumor growth.1
Customer Reviews
-
"Using Creative Biolabs' oncolytic virotherapy development service, we were able to achieve a significant improvement in the specificity of our oncolytic virus for sarcoma cells."
- Dr. A.***onResearch Scientist
-
"Creative Biolabs' expertise in virus engineering and in vivo validation was instrumental in accelerating our preclinical studies and obtaining compelling efficacy data."
- Dr. B.***erSenior Scientist
-
"The customized oncolytic viruses provided by Creative Biolabs demonstrated enhanced anti-tumor activity in our models, allowing us to progress more confidently towards clinical trials."
- Prof. C.***rkPrincipal Investigator
Introduction of Sarcomas
Sarcomas are a diverse group of tumors that originate from mesenchymal tissue, the tissue that forms the body's supporting structures. This means sarcomas can arise in various locations, including bone, muscle, fat, blood vessels, and other connective tissues. Unlike carcinomas, which originate in epithelial cells, sarcomas are relatively rare, comprising only about 1% of all solid tumors. Despite their rarity, sarcomas present significant clinical challenges due to their heterogeneity and tendency for aggressive growth.
Current treatment options for relapsed and metastatic sarcoma, including surgery, radiation, and chemotherapy, have limitations. Surgery, while effective for localized disease, is often not feasible for advanced or metastatic stages. Radiation therapy can help control local diseases but may have significant side effects. Cytotoxic chemotherapy, while used in advanced cases, has limited efficacy for many sarcoma subtypes.
Oncolytic virotherapy is a promising approach that utilizes modified viruses to selectively target and destroy cancer cells, including those of sarcomas.
Oncolytic Virotherapy for Sarcomas
Several oncolytic viruses have been studied in preclinical and clinical trials for the treatment of sarcomas. Ads, HSV, and VV have been genetically modified to target sarcoma cells and trigger an anti-tumor response. Reovirus, an innocuous ds-RNA virus, has also shown activity across a variety of sarcoma types in clinical trials.
- OBP-301 is an oncolytic adenovirus with telomerase-specific replication activity, and the expression of the adenoviral E1A and E1B genes is regulated by the tumor-specific hTERT promoter. Treatment with OBP-301 enhanced the sensitivity of osteosarcoma cells to chemotherapeutic agents.
- HSV-1: Three ongoing clinical trials are evaluating the role of T-VEC in sarcomas, which can be treated in combination with radiation therapy and monoclonal antibodies. And to evaluate the efficacy of intratumoral administration of T-VEC for locally advanced cutaneous angiosarcoma, epithelioid sarcoma, and UPS.
- ADV: oncolytic adenovirus Δ24-RGD showed potent anti-sarcoma activity in osteosarcoma cell lines both in vitro and in vivo when combined with cisplatin. At the same time, a significant reduction in tumor load and good safety were observed in the animal model of osteosarcoma in situ.
- An ongoing sarcoma clinical trial using AdAPT-001, an oncolytic replicative type 5 adenovirus equipped with a transforming growth factor beta (TGF-ß) trap, has shown promising tumor elimination.
Creative Biolabs is dedicated to offering you top-tier oncolytic virus development services for sarcoma research. Our group of specialists stands prepared to deliberate on your unique requirements and assist you in formulating a personalized solution.
[Contact Our Team for More Information and to Discuss Your Project]
Reference
- Hatta, Masahiko, et al. "Efficacy of a third-generation oncolytic herpes simplex virus in refractory soft tissue sarcoma xenograft models." Molecular Therapy-Oncolytics 25 (2022): 225-235. DOI: 10.1016/j.omto.2022.04.010. Distributed under Open Access license CC BY 4.0, Some of the pictures were edited and reformatted.
