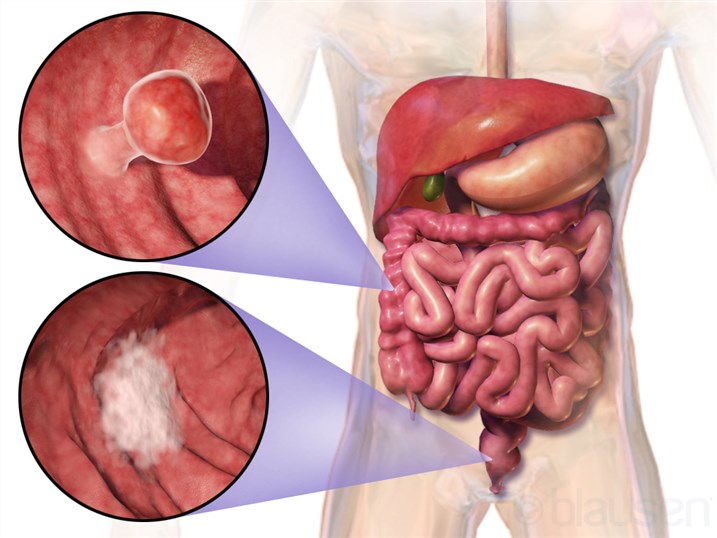
Gastrointestinal Cancer Specific Oncolytic Virotherapy Development Service
Introduction
Gastrointestinal Cancers are serious disorders that impact the digestive tract (GI tract) and the organs involved in the digestive process, including the esophagus, stomach, biliary system, pancreas, small intestine, large intestine, rectum, and anus. As an innovative treatment method, immunotherapy is emerging as a promising and effective option for treating numerous types of cancer. It has had positive results in different lab and clinical studies. Even though using immune checkpoint inhibitor antibodies that target CTLA-4, PD-1, or PD-L1 (the three key immune checkpoint molecules) has provided real-world benefits in treating gastric cancer, colorectal cancer, and pancreatic cancer, oncolytic virotherapy is quickly becoming another treatment with great promise. Many studies have shown that oncolytic viruses (OVs) are highly effective at taking on and fighting gastrointestinal cancers. With Creative Biolabs's oncolytic virus development platform OncoVirapy™ and our growing knowledge of how tumors work in GI cancers, our researchers can create customized oncolytic virotherapy plans and offer services to build and test oncolytic viruses.
The Common Types and Characteristics of Gastrointestinal Cancers
Tab.1 Common types of gastrointestinal cancers.
| Types of Cancer | Main manifestations |
|---|---|
| Gastric cancer |
|
| Colorectal cancer |
|
| Pancreatic cancer |
|
| Esophageal cancer |
|
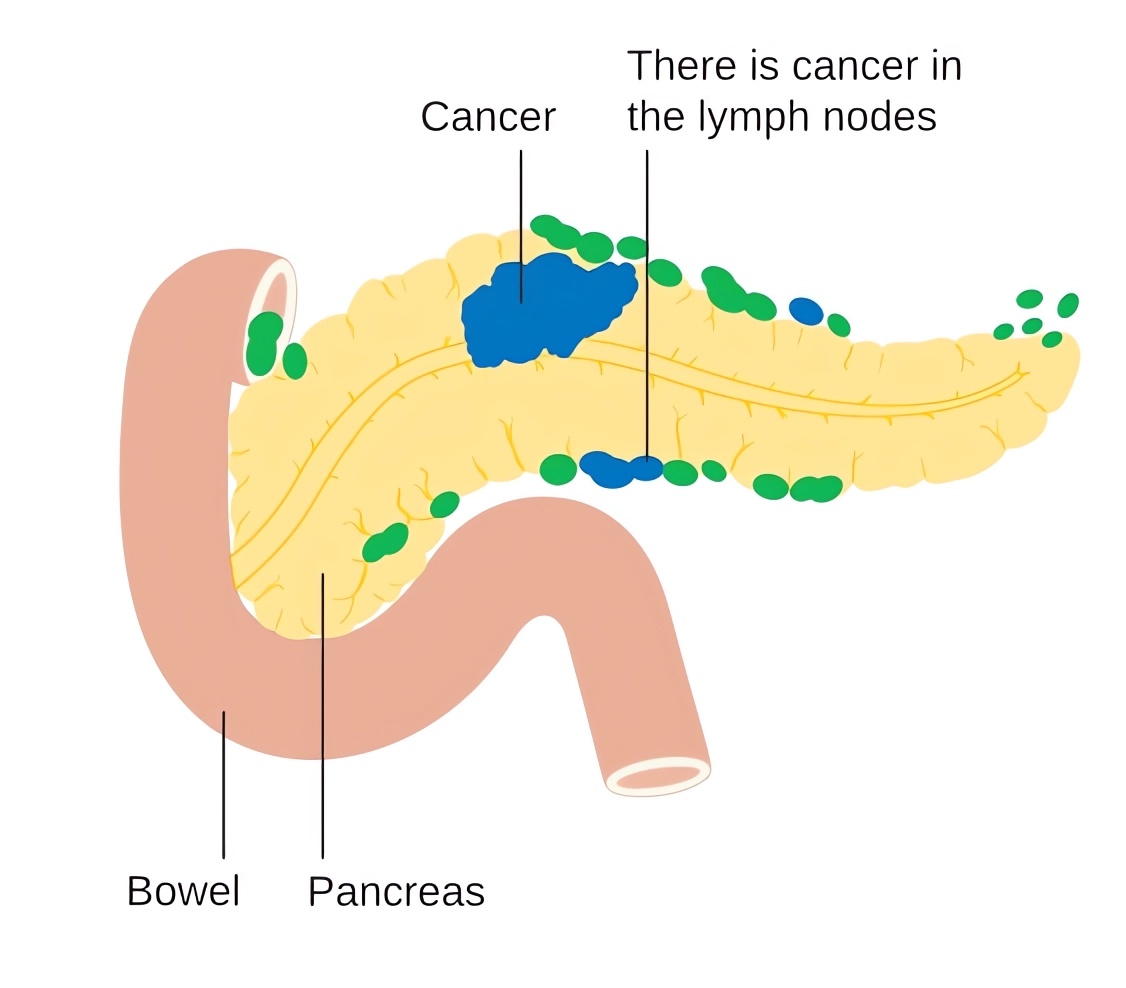 Distributed under CC BY-SA 4.0, from Wiki, without modification.
Distributed under CC BY-SA 4.0, from Wiki, without modification.
Oncolytic Virus Therapy for Gastrointestinal Cancers
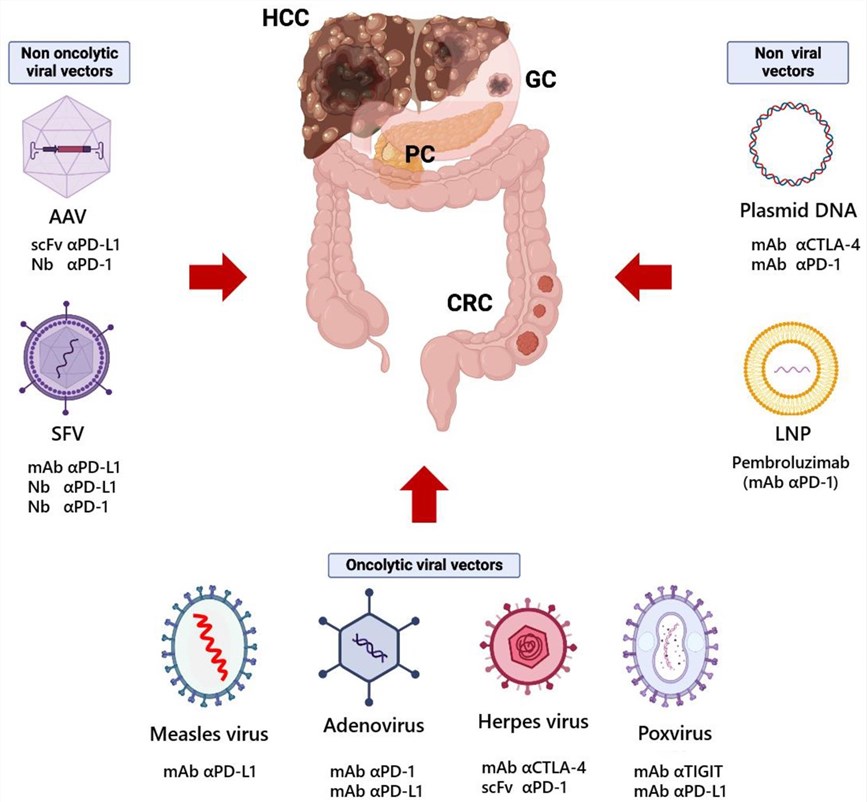 Fig.2 Oncolytic viral vector therapy strategies for gastrointestinal tumors.1
Fig.2 Oncolytic viral vector therapy strategies for gastrointestinal tumors.1
- Pancreatic cancer and Pancreatic ductal adenocarcinomas:
AVL-armed oncolytic cowpox virus boosts replication and anti-tumor ability in pancreatic cancer by raising ROS. The results demonstrated that oncoVV-AVL can enhance the oncolytic effects on pancreatic cancer (PC) cells and induce the production of multiple cytokines, including TNF-α, IL-6, IL-8, and IFN-Ⅰ), without eliciting an antiviral response.
PDAC patients on chemo/radiotherapy have<10% response rate and risk drug resistance. Non-pathogenic OV M1 shows potential for PDAC treatment. It inhibits the viability of different pancreatic cancer cells.
- Gastric cancer
Researchers engineer a novel chimeric orthopoxvirus, CF17, for gastric cancer treatment. In in vivo experiments, intraperitoneal CF17 injection notably decreases peritoneal tumor burden and curbs malignant ascites formation compared to the control.
G47Δ, a third-generation oncolytic HSV-1, is designed by mutating γ34.5, ICP6, and α47 in the HSV-1 genome. Preclinical data show it has strong anti-tumor effects and good safety. In gastric cancer, G47Δ promotes M1 macrophage and NK cell infiltration, reduces M2 macrophages, and holds great promise for research and clinical use.
- Colorectal cancer
V937, a non - transgenic coxsackievirus A21 Kuykendall strain, has undergone clinical evaluation for advanced solid tumor treatment. It specifically targets and lyses tumor cells overexpressing ICAM-1. Additionally, recombinant IFN-γ and pembrolizumab boost ICAM-1 expression, which in turn augments V937-mediated oncolysis and immunogenicity.
The combined oncolytic effects of bortezomib and HSV-1 on colorectal cancer cell lines are evident. This combination controls proteins related to heat stress, stress inside cells (endoplasmic reticulum stress), and cell death (apoptosis). It starts up the caspase-12 process, reduces the level of Bcl-2, and makes cells die off in a programmed way. This effectively stops tumors from growing.
In the face of the demand for innovative therapeutic approaches in gastrointestinal malignancies, oncolytic viruses persist in demonstrating potential as an efficacious treatment option. Creative Biolabs will continuously endeavor to explore this novel methodology to surmount the remaining obstacles and formulate more effective oncolytic virotherapy strategies for its clientele.
Workflow
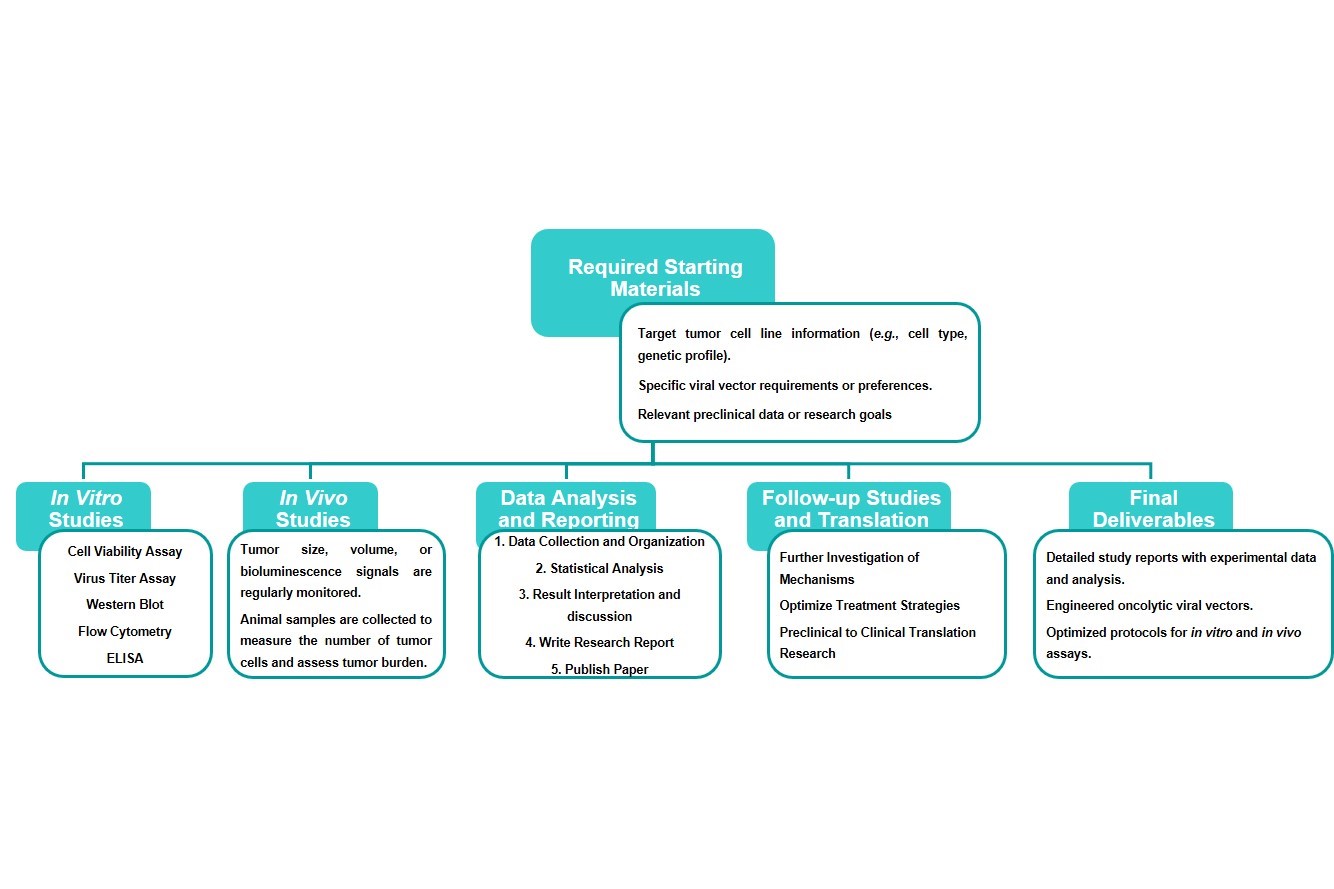
Estimated Timeframe:
Pre-requirement communication: 1-2 weeks
Design and construction of oncolytic viruses: 3-4 weeks
Mass production of oncolytic viruses: 2-3 weeks
Function and properties of oncolytic viruses in vivo and in vitro: 3-4 weeks
Results analysis and test report: 1-2 weeks
Product delivery and shipping: 2-3 weeks
Case Study
The use of genetically engineered oncolytic viruses in widely-applied in vivo mouse models and in vitro cell-line models of gastrointestinal cancers has led to a substantial boost in tumor-destroying potency. Insights culled from numerous scientific studies present important perspectives on its promising future for the treatment of gastrointestinal cancers.
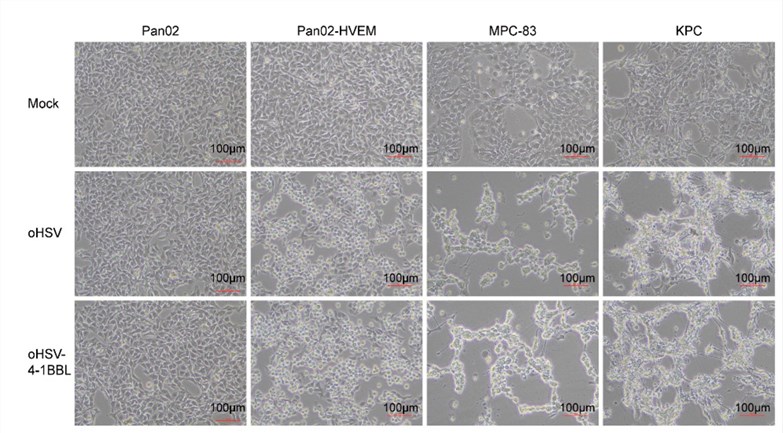 Fig.3 Cytopathic effect of oncolytic virus on tumor cells is observed by microscope.2
Fig.3 Cytopathic effect of oncolytic virus on tumor cells is observed by microscope.2
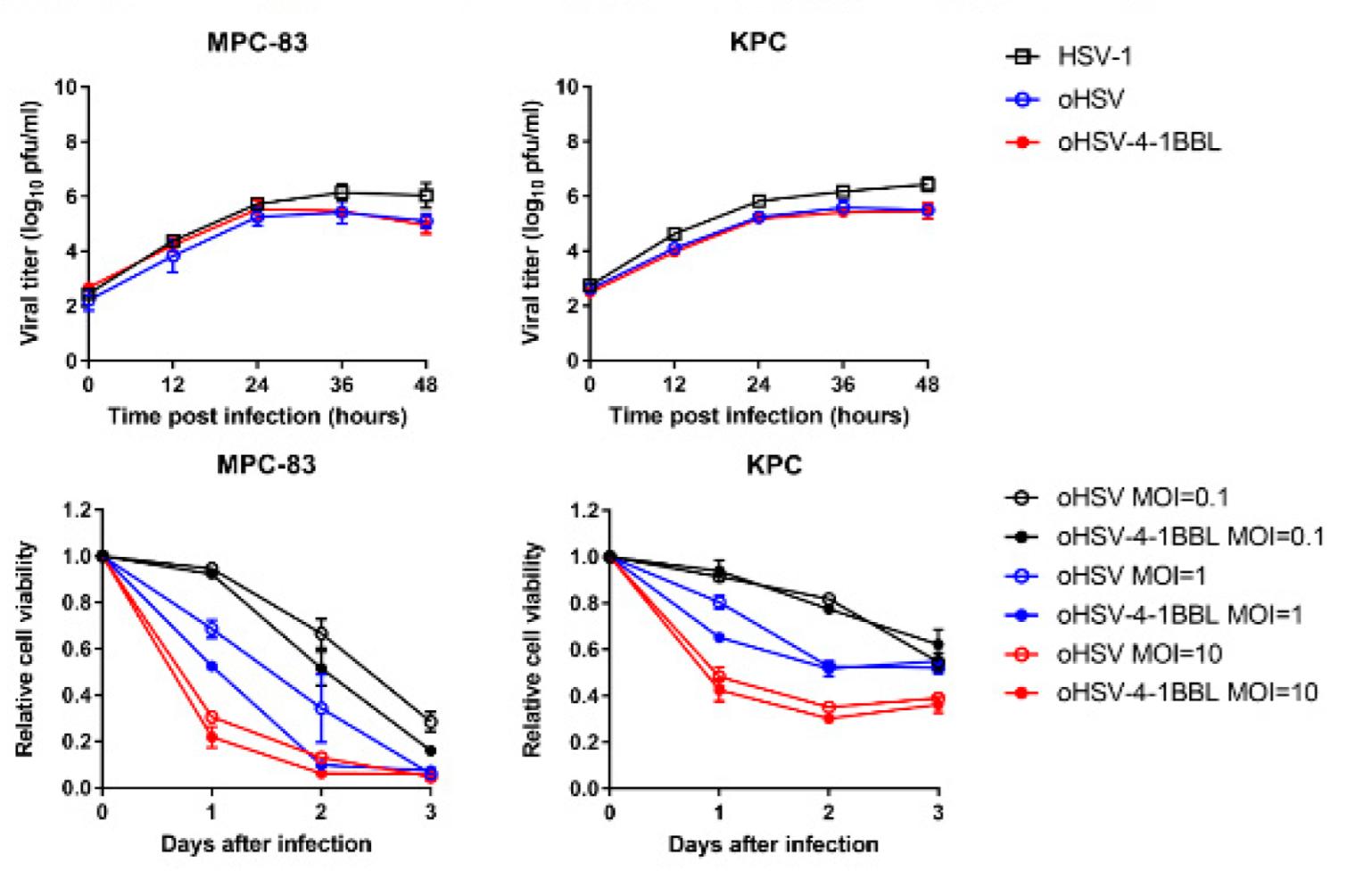 Fig.4 Viral replication kinetics and cell viability after virus infection were detected.2
Fig.4 Viral replication kinetics and cell viability after virus infection were detected.2
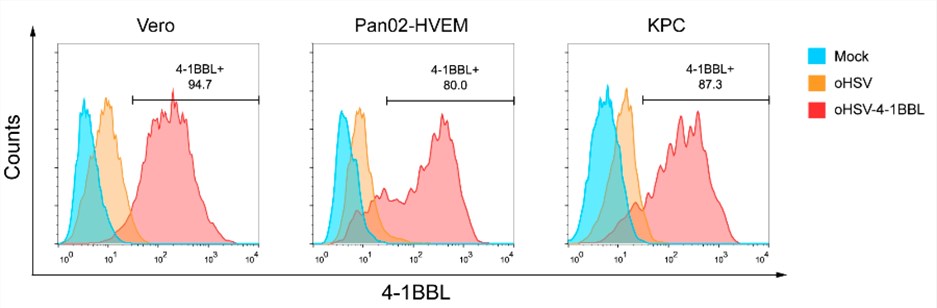 Fig.5 Flow cytometry can be used to detect the expression of related markers.2
Fig.5 Flow cytometry can be used to detect the expression of related markers.2
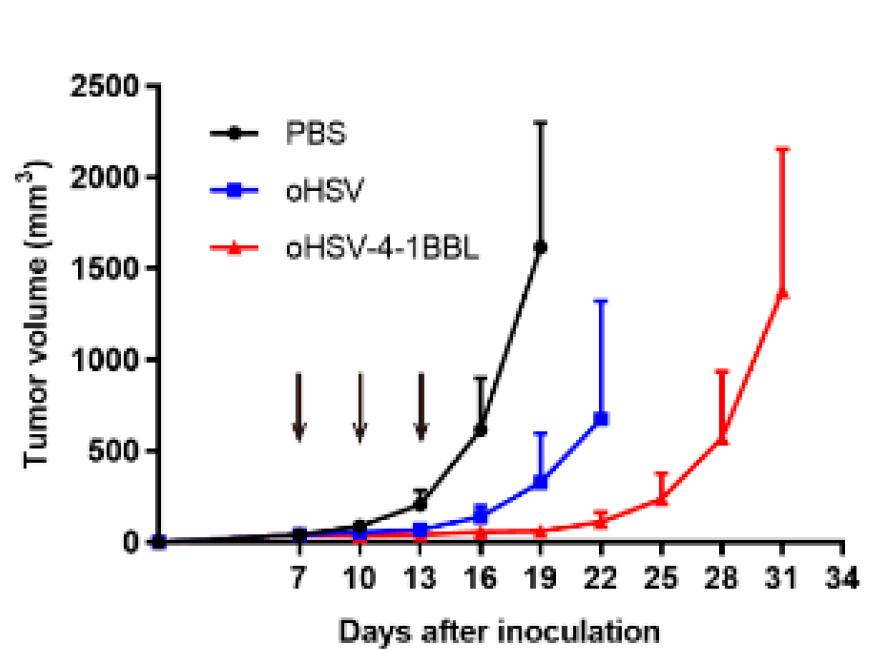 Fig.6 Detection of tumor growth inhibition by oncolytic viruses.2
Fig.6 Detection of tumor growth inhibition by oncolytic viruses.2
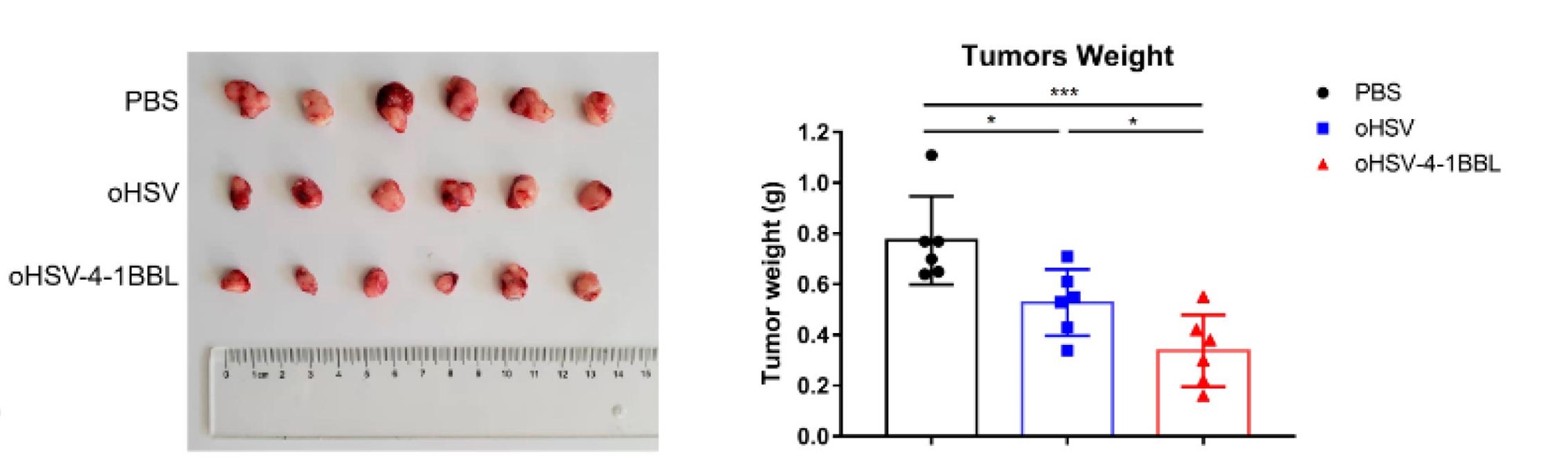 Fig.7 An in vivo tumor model is constructed to detect the effect of an oncolytic virus on tumor size.2
Fig.7 An in vivo tumor model is constructed to detect the effect of an oncolytic virus on tumor size.2
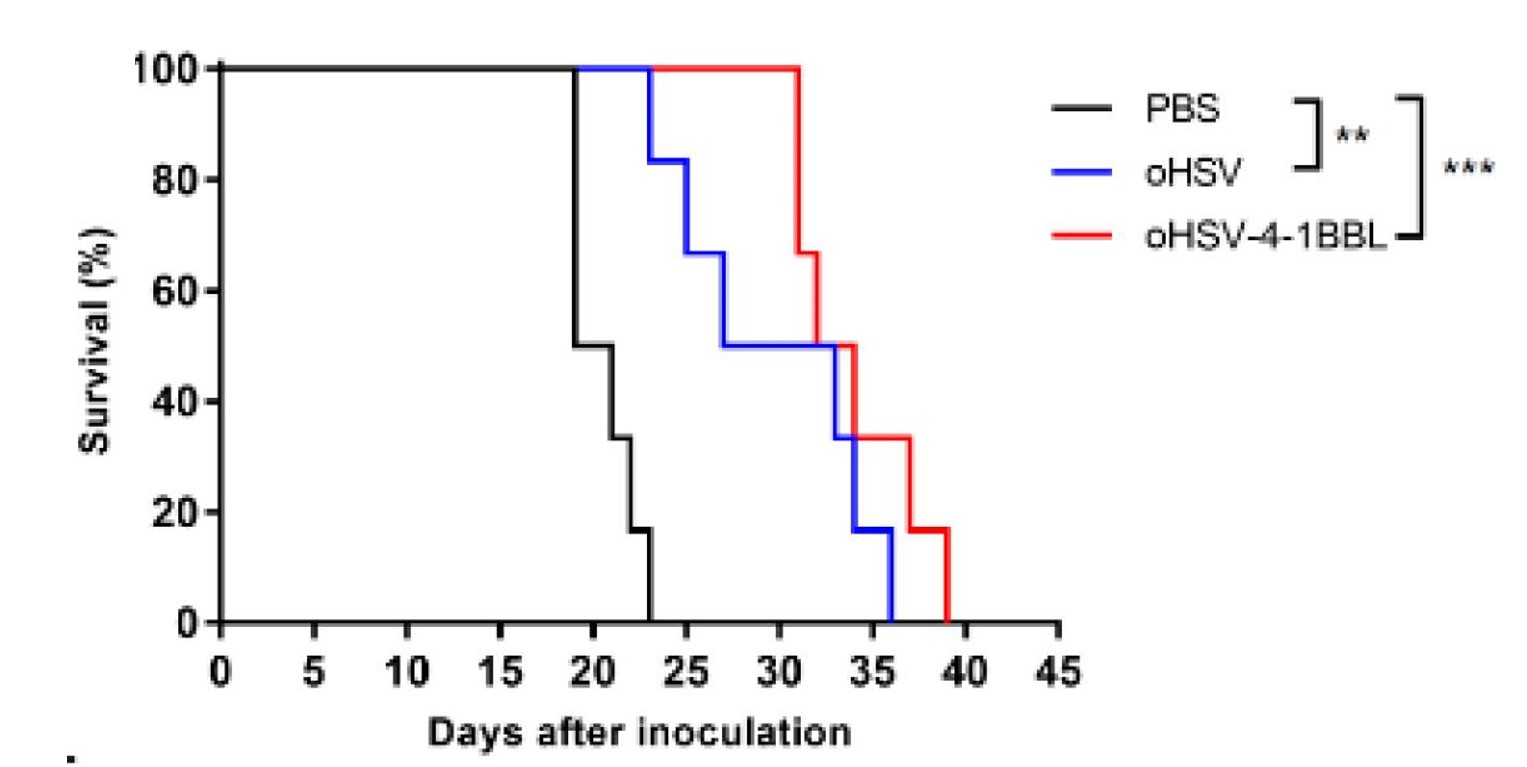 Fig.8 Gene-edited oncolytic viruses prolong Survival of cancer-bearing mice.2
Fig.8 Gene-edited oncolytic viruses prolong Survival of cancer-bearing mice.2
References
- Silva-Pilipich, Noelia, Ángela Covo-Vergara, and Cristian Smerdou. "Local delivery of immunomodulatory antibodies for gastrointestinal tumors." Cancers 15.8 (2023): 2352. DOI: 10.3390/cancers15082352. Distributed under Open Access license CC BY 4.0, without modification.
- Gao, Wenrui, et al. "4-1BBL-Armed Oncolytic Herpes Simplex Virus Exerts Antitumor Effects in Pancreatic Ductal Adenocarcinoma." Vaccines 12.12 (2024): 1309. DOI: 10.3390/vaccines12121309. Distributed under Open Access license CC BY 4.0, without modification, the picture is reformatted.