Chemoradiotherapy & Oncolytic Virotherapy Combination Therapy Development Service
Accelerate Your Drug Discovery Process!
How Creative Biolabs Can Assist Client?
Many researchers face challenges such as long drug development cycles, difficulties in protein expression and purification, obstacles in antibody development, and complex clinical trials. Creative Biolabs' Oncolytic Virotherapy Development for Combination Therapy with Chemoradiotherapy services addresses these issues by leveraging advanced oncolytic virus technologies and combination therapy strategies to accelerate drug discovery and streamline clinical trial processes. The company offers comprehensive oncolytic virotherapy development services, providing tailored solutions to enhance cancer research and drug development projects, and delivering robust and reliable support to help clients navigate the complexities of combination therapy and achieve specific research goals.
Workflow
| Required Starting Materials | Project Consultation and Design |
|---|---|
| To initiate a project, clients typically be asked to provide: Tumor cell line information (e.g., cell type, origin, genetic profile). Specific oncolytic virus strains of interest. Details of chemotherapeutic agents or radiation therapy protocols intended for combination. | We begin with an in-depth consultation to understand your project goals, specific requirements, and any existing constraints. Based on this, we design a customized oncolytic virotherapy development strategy, including virus selection, modification, and combination therapy protocols. |
| In Vitro Efficacy Testing | In Vivo Preclinical Studies |
| We conduct rigorous in vitro studies to evaluate the efficacy of the oncolytic virus and its combination with chemotherapeutic agents or radiation therapy. This includes assessing cell viability, cytotoxicity, viral replication, and synergistic effects. | Creative Biolabs performs preclinical in vivo studies using appropriate animal models to evaluate the safety and efficacy of oncolytic virotherapy. This includes assessing tumor growth inhibition, survival rates, biodistribution, and potential toxicity. |
| Data Analysis and Reporting | Final Deliverables |
| Our team of experts analyzes the experimental data using advanced bioinformatics tools and statistical methods. We provide comprehensive reports summarizing the results, including detailed methodologies, data visualizations, and interpretations. | Clients receive a comprehensive set of deliverables, including: Detailed study reports with experimental data, statistical analyses, and interpretations. Optimized oncolytic virus constructs and protocols. Recommendations for further development or clinical translation. |
| Estimated Timeframe | |
| The typical timeframe for this service is 12 to 20 weeks, factors that may influence the duration include the specific oncolytic virus used, the need for genetic modifications, the complexity of the in vivo studies, and any regulatory requirements. | |
[Discover How We Can Help - Request a Consultation]
Case Study
The application of combination therapy integrating genetically modified oncolytic viruses with radiotherapy or chemotherapy in common in vivo murine models and in vitro gastrointestinal cancer cell line models has resulted in a significant enhancement of anti-tumor efficacy. Findings from numerous published studies provide valuable insights into its great potential for treating gastrointestinal cancers, demonstrating that such combined strategies can effectively boost therapeutic outcomes through synergistic mechanisms.
| Cytotoxicity | Cell Proliferation |
|---|---|
|
|
|
| Evaluation of Oncolytic Virus Combined with Chemotherapy | |
|
|
|
| Flow Cytometry | H&E and Immunohistochemical Staining |
|
|
|
| Nude Mouse Model | Tumor Volume |
|
|
|
Customer Reviews
-
"Using Creative Biolabs' Oncolytic Virotherapy Development services in our research has significantly improved the speed and efficiency of our drug discovery process."
[J**n D**e][3 Months]
-
"Creative Biolabs' expertise in oncolytic virus engineering was invaluable in overcoming challenges we faced in achieving effective tumor-specific targeting."
[M**k S**h][6 Months]
-
"The comprehensive data analysis and reporting provided by Creative Biolabs enabled us to make informed decisions and accelerate our preclinical development timeline."
[S**n K**y][9 Months]
[Experience the CB Advantage - Get a Quote Today]
What We Can Offer
- Customized Oncolytic Virus Design and Construction: We engineer oncolytic viruses tailored to your specific tumor targets and therapeutic goals.
- Comprehensive Preclinical Evaluation: We conduct in vitro and in vivo studies, including efficacy testing and safety profiling, adhering to the highest scientific standards.
- Combination Therapy Optimization: We design and optimize oncolytic virus-based combination therapies with chemotherapeutic agents or radiation therapy to maximize synergistic effects.
- Regulatory Support: We provide expert guidance and support to navigate the regulatory requirements for oncolytic virotherapy development.
- Advanced Analysis and Quality Assurance: We utilize cutting-edge analytical methods to guarantee the quality, purity, and efficacy of our oncolytic virus products.
- Dedicated Project Management: We assign a dedicated project manager to ensure seamless communication, efficient execution, and on-time delivery of your project.
Why Choose Us?
- Expertise: Creative Biolabs possesses deep expertise in oncolytic virus biology, cancer biology, and combination therapy strategies. Our team has a proven track record of successfully developing and optimizing oncolytic virotherapies for various cancer types.
- Comprehensive Platform: Creative Biolabs offers a comprehensive platform that includes a wide range of oncolytic viruses, genetic engineering tools, and preclinical models. This allows us to tailor our services to your specific needs and accelerate your research.
- Innovative Technology: Creative Biolabs employs the most advanced technologies and approaches to ensure the superior quality and efficiency of our services. We are dedicated to remaining at the leading edge of oncolytic virotherapy research.
- Customized Solutions: Creative Biolabs understands that every project is unique. We work closely with our clients to develop customized solutions that meet their specific requirements and address their unique challenges.
- Proven Success: Our clients experience significant improvements in their research outcomes.
Creative Biolabs is dedicated to formulating rational and synergistic combination strategies that meet our clients' demands and accelerate the development of effective cancer treatments. If you have any need, please feel free to contact us!
Reference
- Mori, Yoshinori, et al. "Modulation of Reoviral Cytolysis (I): Combination Therapeutics." Viruses 15.7 (2023): 1472. DOI: 10.3390/v15071472. Distributed under Open Access license CC BY 4.0, Some of the pictures were edited and reformatted.

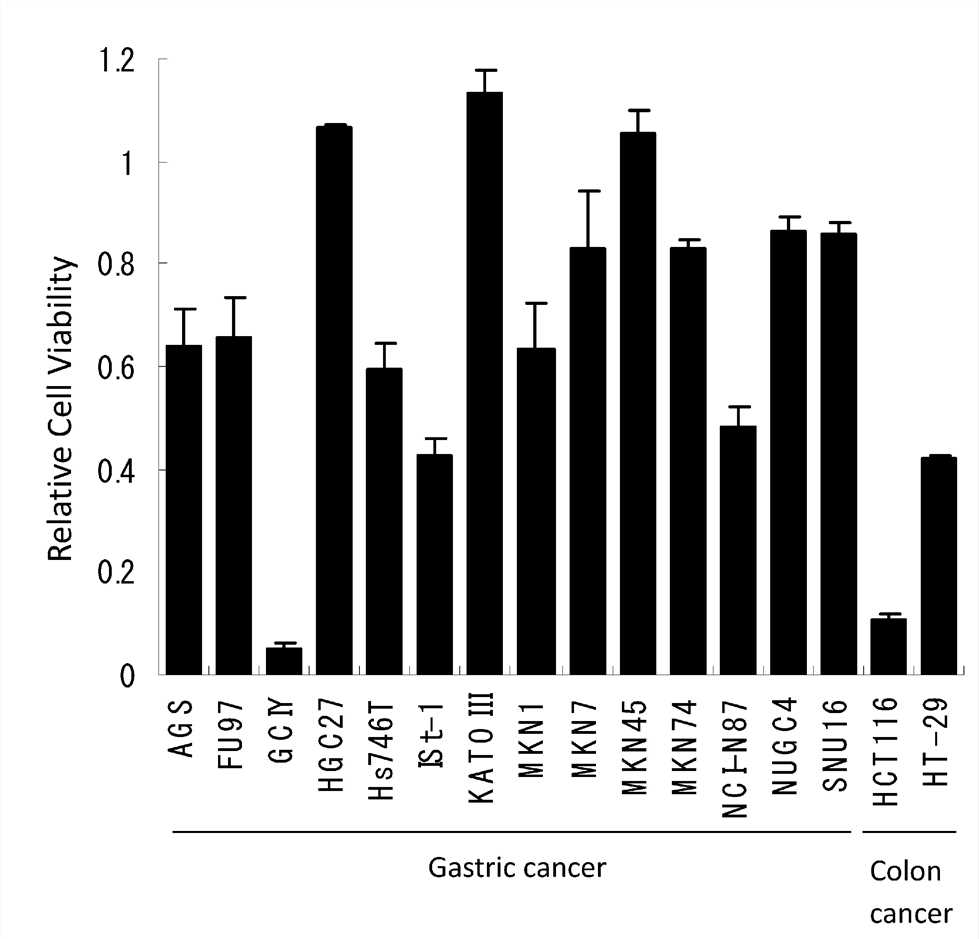 Fig.1 The cytotoxicity of oncolytic viruses is assessed by WST-1 assays.1
Fig.1 The cytotoxicity of oncolytic viruses is assessed by WST-1 assays.1
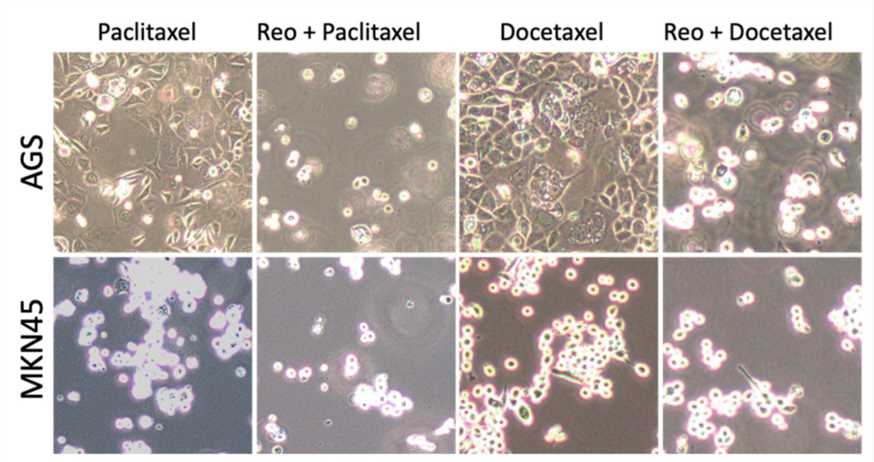 Fig.2 The effect of the oncolytic virus on the proliferation of tumor cells is observed under a microscope.1
Fig.2 The effect of the oncolytic virus on the proliferation of tumor cells is observed under a microscope.1
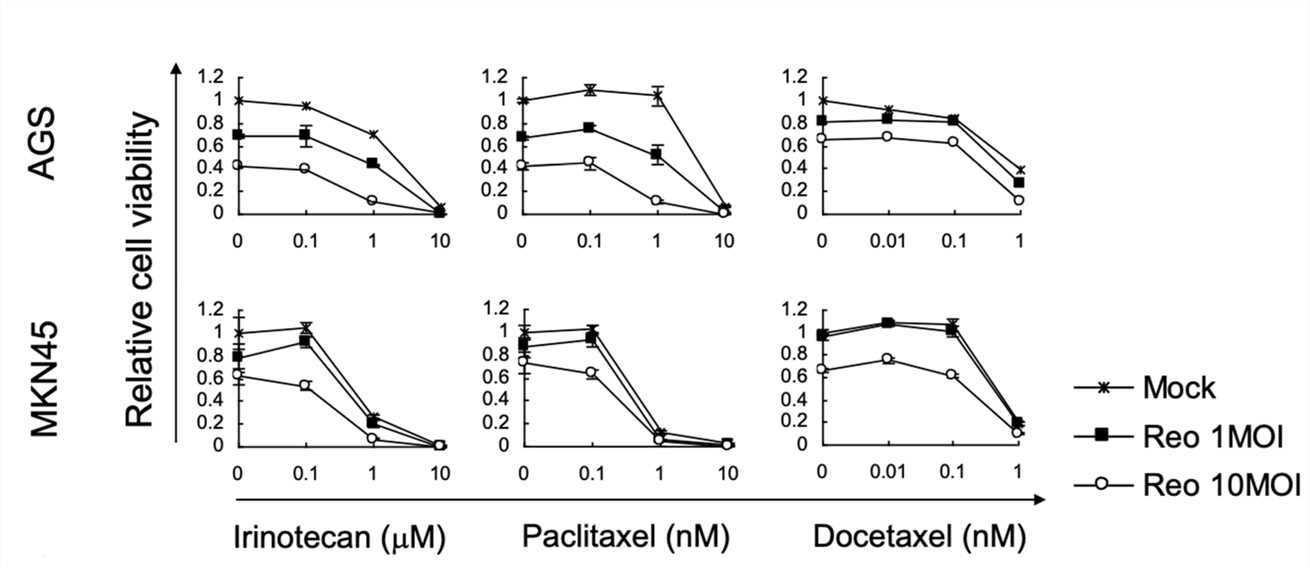 Fig.3 WST-1 assay is used to evaluate the synergistic effect of chemotherapy drugs and oncolytic viruses.1
Fig.3 WST-1 assay is used to evaluate the synergistic effect of chemotherapy drugs and oncolytic viruses.1
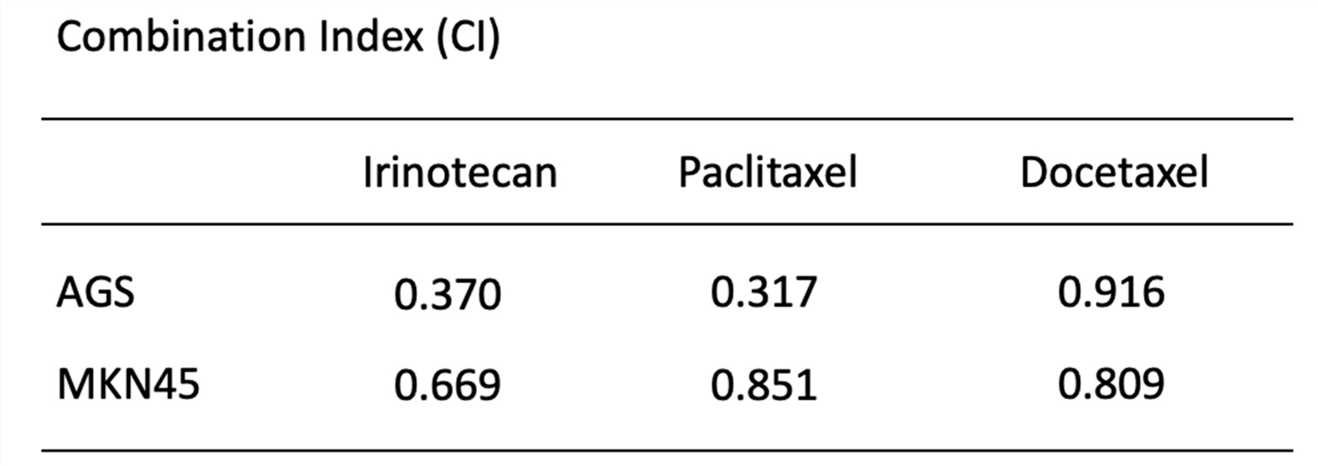 Fig.4 Calculation of combination index.1
Fig.4 Calculation of combination index.1
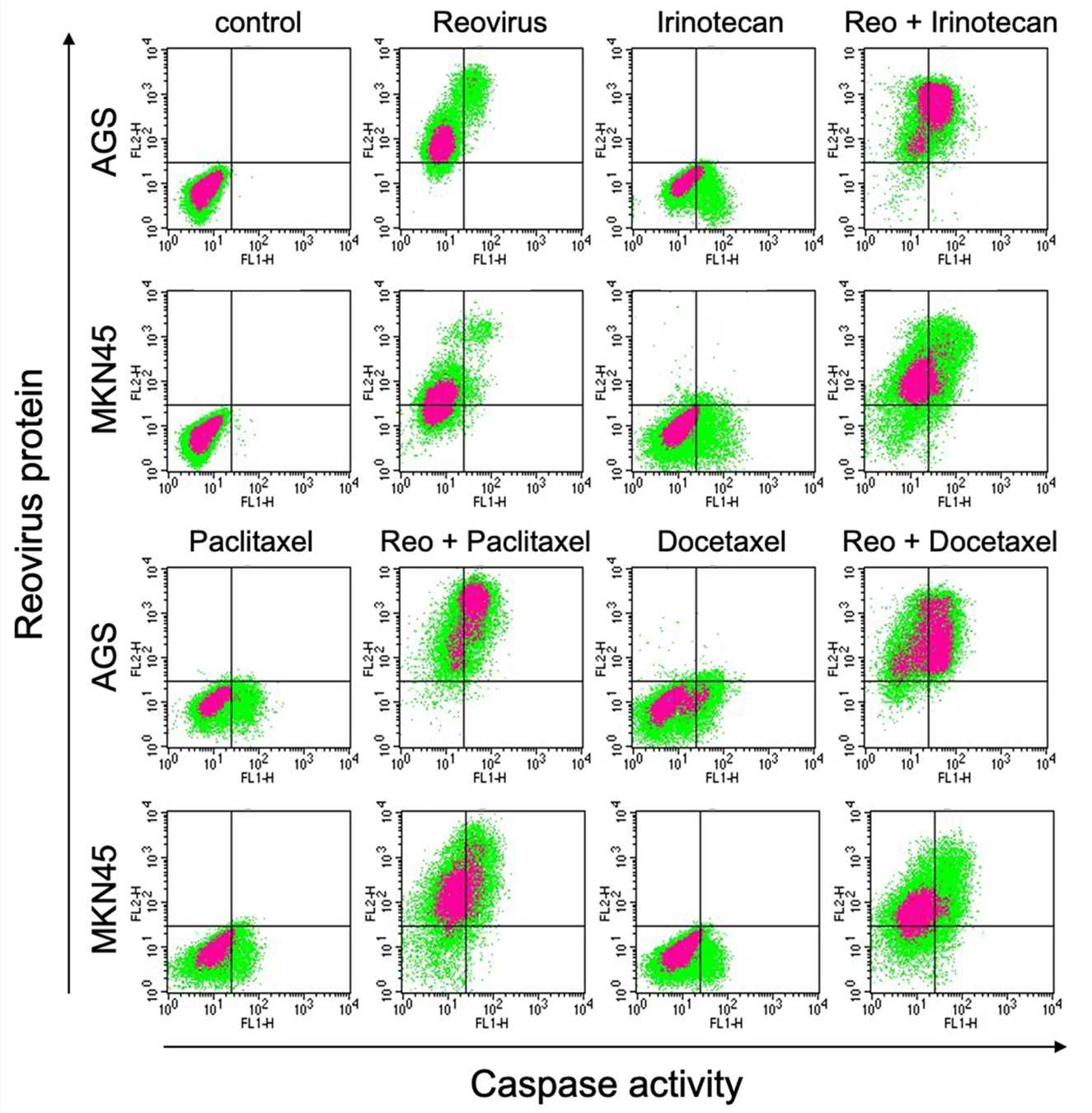 Fig.5 The combination therapy promotes the apoptosis of tumor cells.1
Fig.5 The combination therapy promotes the apoptosis of tumor cells.1
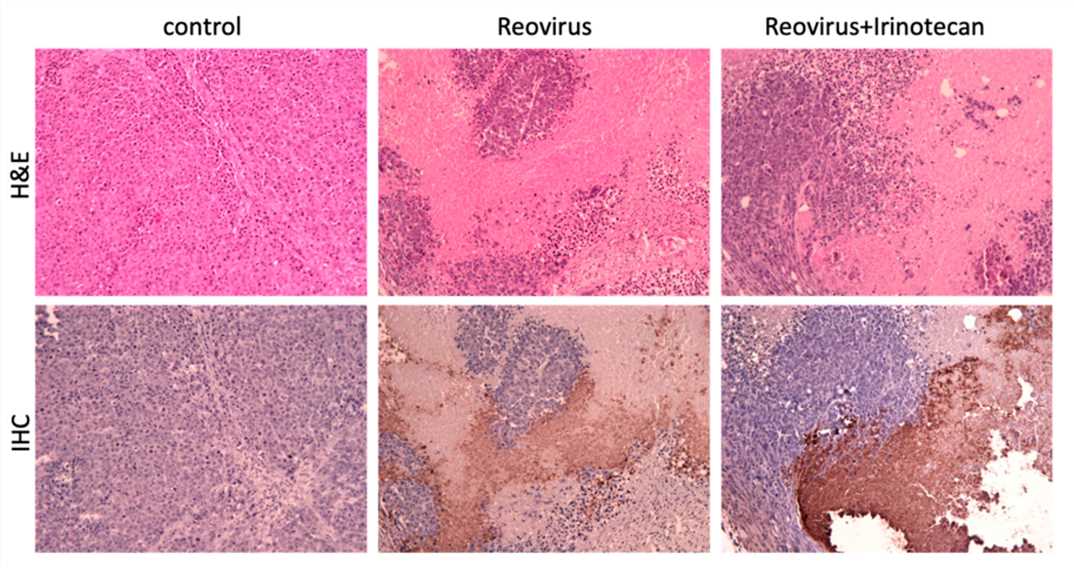 Fig.6 Wide distribution of viruses in tumors.1
Fig.6 Wide distribution of viruses in tumors.1
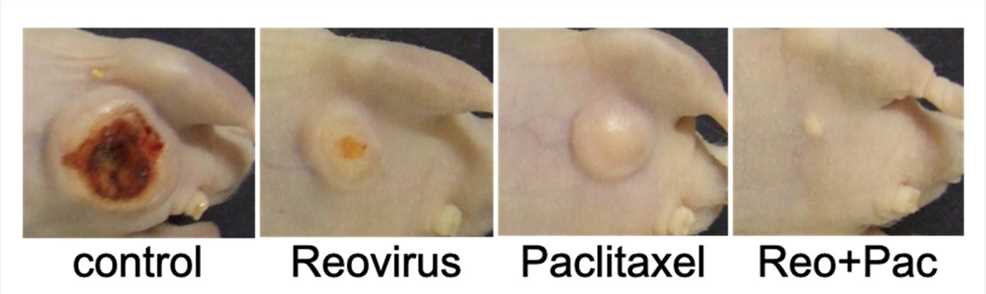 Fig.7 Combination therapy limited tumor growth.1
Fig.7 Combination therapy limited tumor growth.1
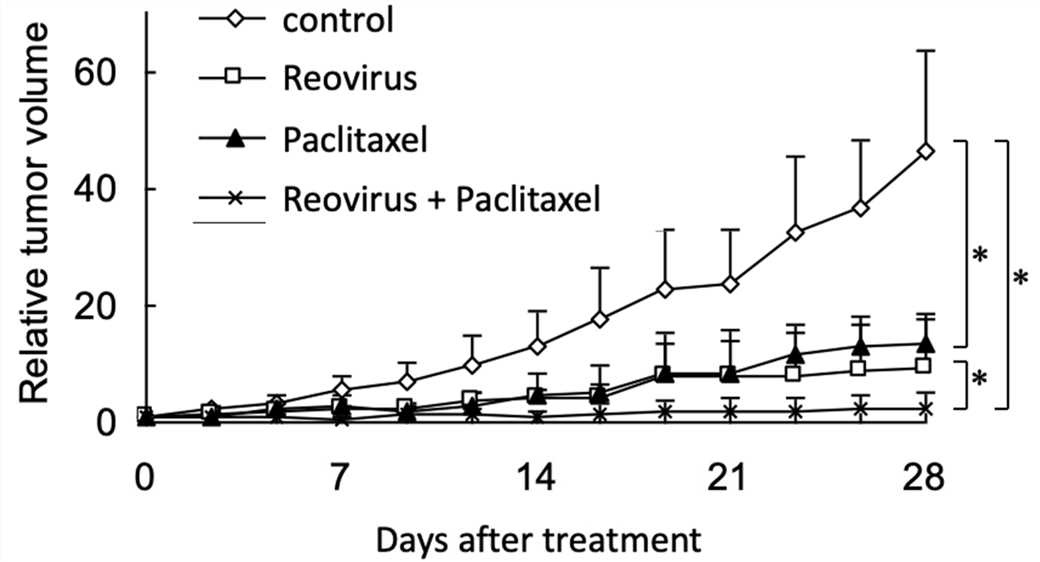 Fig.8 Combination therapy delays the increase in tumor volume.1
Fig.8 Combination therapy delays the increase in tumor volume.1