Oncolytic Virus Efficacy Study Service
The measurement of oncolytic virus efficacy is crucial. It directly evaluates therapeutic effects, clarifying their role in inhibiting tumor growth, inducing apoptosis, and shrinking tumors, thus providing a vital basis for therapy feasibility. Also, it determines optimal dosage, administration route, and treatment course, guiding clinical application and treatment plan optimization. Creative Biolabs' professional team can comprehensively assess virus efficacy in animal models per client needs.
Experimental Methods for Studying Oncolytic Virus Efficacy
- Oncolytic Efficacy Analysis
The inhibitory effect of oncolytic viruses on tumor growth was evaluated by measuring tumor volume, recording the survival time of animals, and calculating the T/C ratio. Employing small-animal in vivo imaging techniques, such as fluorescence imaging and bioluminescence imaging, the growth of tumors and the in vivo distribution and replication of oncolytic viruses were observed in real time.
- Immunological Analysis
Analysis of Immune Cell Changes: The alterations in the numbers of various immune cells, including T cells, B cells, NK cells, and macrophages, along with immune-related markers and their infiltration in tumor tissues, were detected using flow cytometry, immunohistochemistry, immunofluorescence, and HE staining techniques. Subsequently, the types, quantities, and distributions of these immune cells were analyzed to assess the impact of oncolytic viruses on the recruitment and activation of immune cells within the tumor microenvironment.
Analysis of Immune Cell Function Changes: Blood samples from animals and homogenates of their tumor tissues are collected. The expression levels of diverse cytokines, including interferon, interleukin, and tumor necrosis factor, are detected using ELISA, liquid chip technology, flow cytometry, and other techniques. This is done to elucidate the role of oncolytic viruses in the body's immune regulation.
- Transgene Expression Analysis
Through gene editing, oncolytic viruses can have their functions significantly optimized, enabling them to play a more effective role in anti-tumor therapy. To further investigate the characteristics of gene-edited oncolytic viruses, a diverse range of advanced molecular biology (e.g., ELISA, RT-PCT, PCR, western blot, Flow cytometry) and cell biology techniques can be employed to comprehensively examine the expression of their transgenes, thereby effectively validating the efficacy of oncolytic viruses.
- Histological and Pathological Analysis
Tab.1 Commonly used histological and pathological experimental techniques.
| Hematoxylin-eosin (HE) Staining | Immunohistochemical Staining |
|---|---|
|
|
|
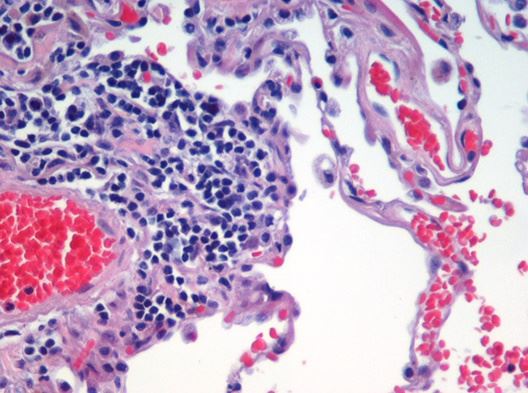
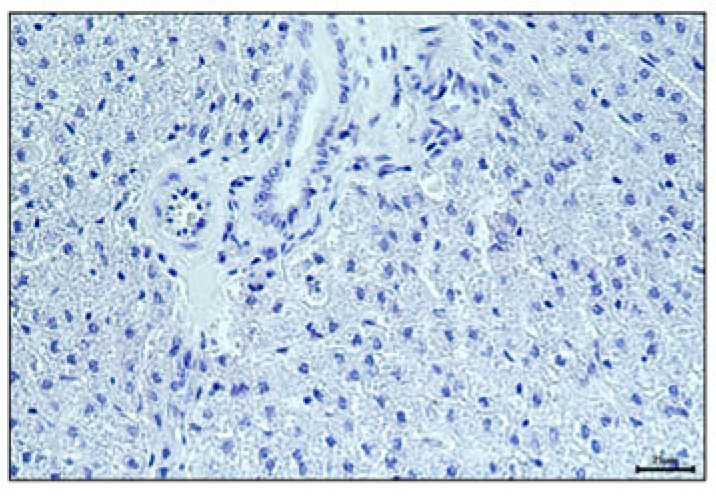
| After HE staining, the morphological structure, cell morphology, and characteristics of the nucleus and cytoplasm in tumor tissue could be observed. Moreover, the necrosis of tumor cells and the infiltration of inflammatory cells could be analyzed. | By leveraging the principle of antigen-antibody-specific binding, antibodies targeting specific proteins are utilized to label target proteins in tumor tissue. This approach enables the assessment of tumor cell proliferation activity and markers associated with immune cells. |
| In situ Hybridization | Special Staining Techniques |
|
|
|
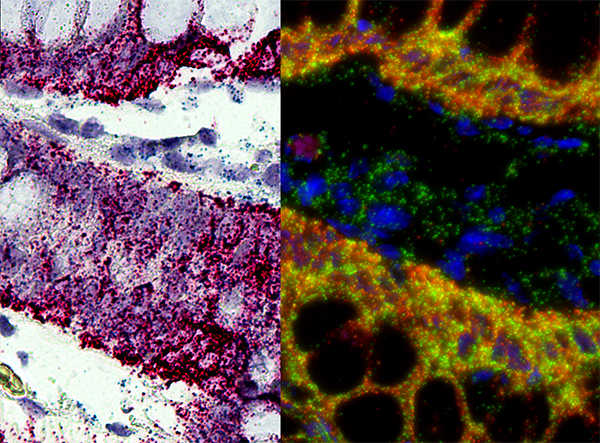
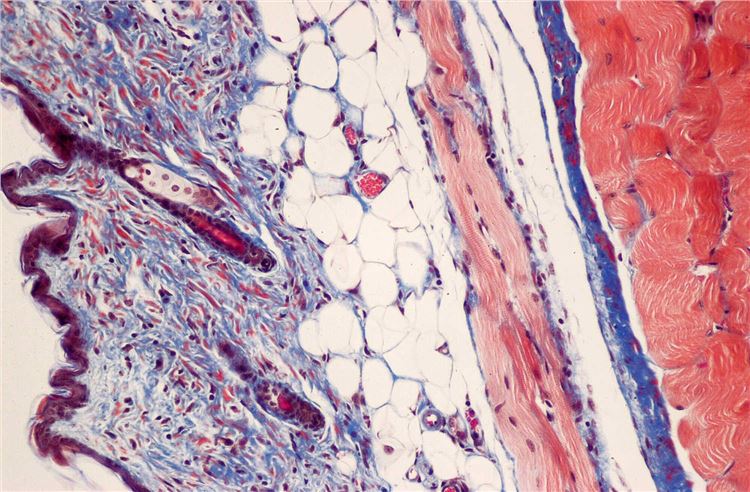
| The distribution of the virus in tumor tissue and the replication of the virus can be determined. | Masson's trichrome staining determines tumor fibrosis degree. PAS staining reveals intracellular glycogen and other components, aiding the analysis of tumor cell metabolic alterations. |
Reference
- Zabelina, Daria S., et al. "Ad6-Based GM-CSF Expressing Vector Displays Oncolytic and Immunostimulatory Effects in an Immunocompetent Syrian Hamster Model of Cholangiocarcinoma." Viruses 17.2 (2025): 162. Distributed under Open Access license CC BY 4.0, a part of the B is intercepted and applied.