Oncolytic Adenovirus Construction Services
Oncolytic adenovirus (Ads) is the most commonly used oncolytic virus (OV) in clinical studies, and it is also the first commercialized oncolytic viral drug. By reconstructing the genome of oAds, it can effectively avoid unwanted cell or tissue damage, and ensure effective anti-cancer efficacy and cancer selectivity. With years of experience in OV development, Creative Biolabs commits to providing clients with professional and customized oAds construction strategies.
Introduction of Adenovirus
Ads belong to the family Adenoviridae. Ads are divided into seven subspecies from A to G and more than 110 genotypes. Ads are non-enveloped viruses with an icosahedral structure and contain a 36-38 kb size double-stranded DNA. The genome can be divided into early genes (E1A, E1B, E2B, E2A, E3, E4) and late genes (L1, L2, L3, L4, L5)1. When adenovirus infects and internalizes into target cells, early genes begin to be expressed, regulating the genes that are required for adenoviral protein synthesis and replication, and late genes begin to be expressed after viral replication, and are associated with cell lysis.
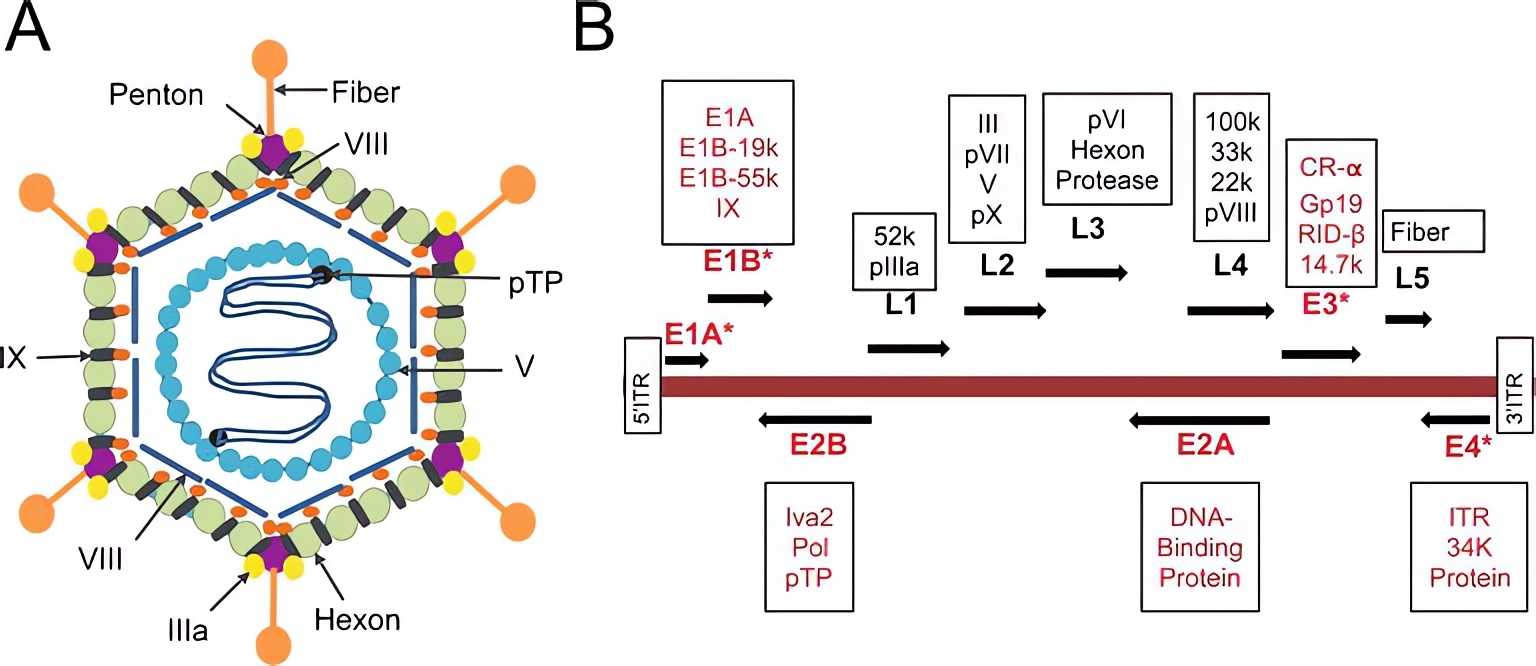 Fig.1 The structure and the genome organization of Adenovirus.2
Fig.1 The structure and the genome organization of Adenovirus.2
The capsid and the genome constitute the adenovirus structure. Hexon, penton, fiber, pⅢa, pⅧ, and pⅨ constitute the capsid, modification of which can reduce the immunogenicity of Ads.
Human Adenovirus Classification and Tropism
Different subspecies of Ads dependent on different cellular receptors, the subspecies A, C, E, and F rely on the coxsackievirus and adenovirus receptors (CAR), the subspecies B and D bind to CD46, CD86, CD80, and DSG-2, integrin ανβ3/5 is used as the secondary receptor of all the subspecies of Ads. Ad2 and Ad5, both subspecies C, are commonly used gene therapy tools. Ads can spread through the respiratory tract, causing gastrointestinal and respiratory symptoms and conjunctivitis.
Tab.1 The subspecies, serotypes, receptors, and associated disease of human adenovirus.
| Species | Serotypes | Receptors | Disease |
|---|---|---|---|
| A | 12, 18, 31 | hCAR | Obesity or adipogenesis |
| B | 3, 7, 11, 14, 16, 21, 34, 35, 50, 55 | CD46, CD80, CD86, DSG-2 | Respiratory disease, conjunctivitis |
| C | 1, 2, 5, 6, 5, 7 | hCAR, HSPG, VCAM-1, SR, MHC-1-α2 | Respiratory disease, obesity, or adipogenesis |
| D | 8-10, 13, 15, 17, 19, 20, 22-30, 32, 33, 36-39, 42-54, 56, 58-60, 62-65, 67, 69-75 | hCAR, SA, CD46, GD1a/Sialic acid | Conjunctivitis, obesity or adipogenesis |
| E | 4 | hCAR | |
| F | 40, 41 | hCAR | Gastroenteritis |
| G | 52 | nd | Gastroenteritis |
Genetic Modifications of Oncolytic Adenovirus Vectors
- Strategy design to improve the safety of OAVs
Through precise modification of the Ads genome, it is possible to achieve the attenuation of adenoviruses and their specific replication within tumor cells. When the early genes E1A, E1B, and E3 of Ads are moderately deleted or engineered (such as deleting the E1A-CR2 region, E1B-19k, E1B-55k, E3-gp19k, etc.), the replication of Ads in normal cells can be effectively suppressed, thereby enhancing safety. By introducing a tumor tissue-specific promoter (TSP) to replace the native Ads promoter, specific replication of Ads within tumor cells can be accomplished. TSPs include those that are expressed in all tumors, such as the survivin promoter, COX-2 promoter, and human telomerase reverse transcriptase (hTERT) promoter, as well as promoters specific to certain tumors, such as prostate-specific antigen (PSA), alpha-fetoprotein (AFP), carcinoembryonic antigen (CEA).
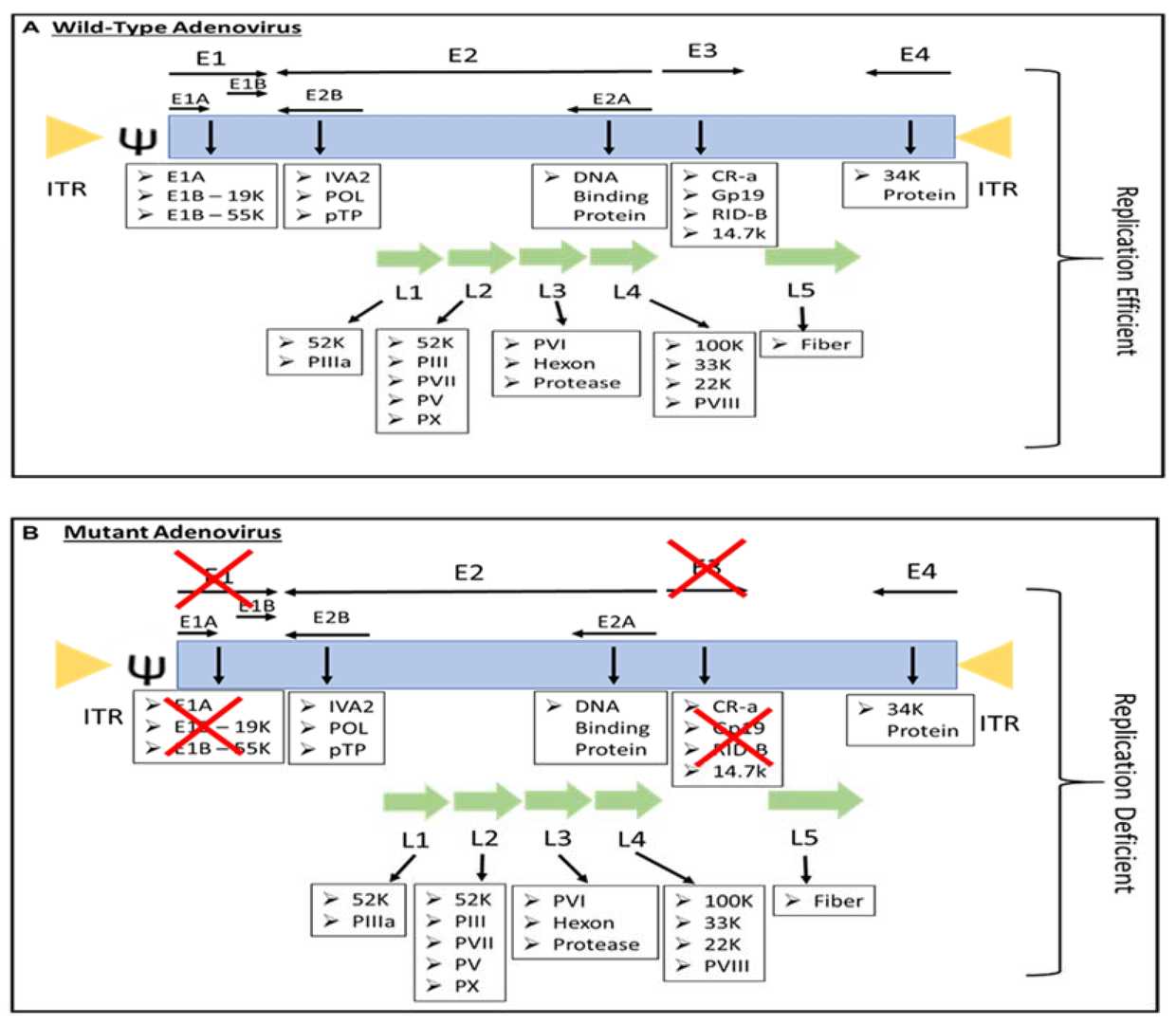 Fig.2 Genomic differences between wild type and mutant Ads.3
Fig.2 Genomic differences between wild type and mutant Ads.3
-
Strategic design of OAVs targeting tumor
- Ads armed with cytokines and chemokines: GM-CSF, IL-12, and IL-2 have been developed for modification of Ads, which can promote the activation of immune cells and inhibit tumor proliferation.
- Ads armed with co-stimulatory molecule: Ads expressing CD40L (Ad3-hTERT-CMV-hCD40L) enhance the activation of DC cells.
- Ads armed with immune checkpoint inhibitor: Usually the Ads encode anti-CTLA-4 or anti-PD-L1.
- Ads armed with the bispecific antibodies: Freedman et al. arm Ads with a bispecific antibody, which adhere to epithelial cell adhesion molecules (EpCAM) on target cancer cells and bridge them to CD3 on T cells. This construct effectively aggregates and activates both CD4⁺ and CD8⁺ T cells4.
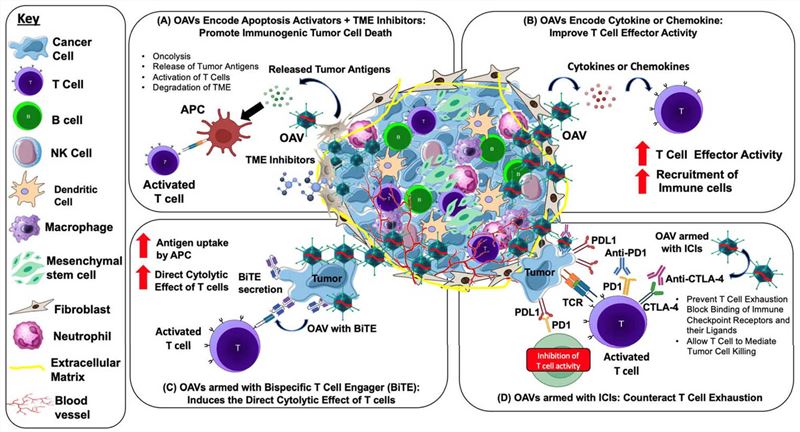 Fig.3 The methods to enhance the targeting of oAds to tumors.3
Fig.3 The methods to enhance the targeting of oAds to tumors.3
Featured Services and Construction Workflow
Based on our advanced OncoVirapy™ platform, we provide professional oncolytic adenovirus construction and production services.
- Select the GOI (encoding antibodies, cytokines, chemokines or immune checkpoint inhibitors, etc.) or the genes plan to be deleted for restriction mapping and sequencing verification
- Functional validation of transgenes
- Expression cassette design with the most appropriate promoter to guarantee the high-level expression
- Viral genes engineering (E1A, E1B, E3)
- Capsid (fiber, penton, hexon) modification to alter the Ad tropism
- Different construction strategies
- Ad amplification, purification, titering services
- Ad whole genome sequencing (WGS) service
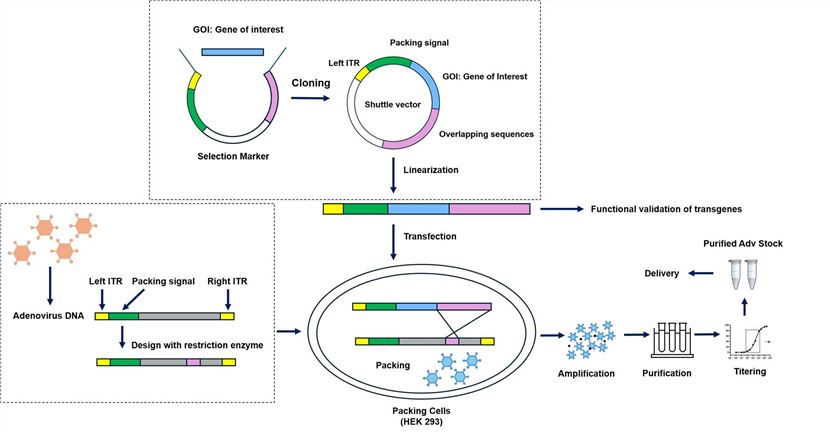 Fig.4 Workflow for Creative Biolabs construction of oncolytic adenoviruses.
Fig.4 Workflow for Creative Biolabs construction of oncolytic adenoviruses.
Cases of Adenovirus Genetic Modifications
- H101 is the first commercialized Ad, which uses genetic engineering technology to knock out the E1B-55KD gene fragment and part of the gene fragment in the E3 region of wild-type Ad5
- Δ24-RGD Oad is a p16/Rb pathway selective Ad with RGD-4C modification of fibers characterized by the control of GM-CSF by the adenovirus E3 promoter
- OBP-301 is an attenuated Ad5 armed with the hTERT promoter for increased adenovirus expression
- VT-01 is used to treat invasive bladder cancer and has entered the clinical trial stage
- CG0070 expresses E2F-1 and GM-CSF, which can selectively replicate in Rb-deficient tumor cells and eventually lyse tumor cells, thereby releasing tumor antigens and GM-CSF.
- YSCH-01 is an Ad carrying an interferon-like immune anti-cancer gene (L-IFN), which can directly kill tumor cells and stimulate the immune regulation of the immune system.
As an emerging force in the field of tumor therapy, oncolytic adenovirus is being explored and studied continuously. By optimizing the construction strategy of oncolytic adenovirus, the potential side effects of oncolytic adenovirus can be effectively reduced and its targeted killing ability to tumor cells can be enhanced. With extensive industry experience, Creative Biolabs provides comprehensive and customized oncolytic adenovirus cloning and construction services. In addition, we provide a one-stop service for oncolytic adenoviruses from in vitro cell experiments to in vivo animal model validation. If you have any questions or needs regarding oncolytic adenovirus construction services, please feel free to contact us and we will be glad to serve you.
References
- Enow, Junior A., Hummad I. Sheikh, and Masmudur M. Rahman. "Tumor tropism of DNA viruses for oncolytic virotherapy." Viruses 15.11 (2023): 2262.
- Singh, Shakti, Rakesh Kumar, and Babita Agrawal. "Adenoviral vector-based vaccines and gene therapies: current status and future prospects." Adenoviruses 4 (2019): 53-91. Distributed under Open Access license CC BY 3.0, without modification.
- Tan, Ee Wern, et al. "Engineered oncolytic adenoviruses: an emerging approach for cancer therapy." Pathogens 11.10 (2022): 1146. Distributed under Open Access license CC BY 4.0, without modification.
- Freedman, Joshua D., et al. "Oncolytic adenovirus expressing bispecific antibody targets T‐cell cytotoxicity in cancer biopsies." EMBO molecular medicine 9.8 (2017): 1067-1087.
