Hematologic Malignancy Specific Oncolytic Virotherapy Development Service
Substantial scientific impediments persistently hinder the development of efficacious therapeutics for hematologic malignancies. Our specialized Oncolytic Virotherapy Development Services are precisely engineered to provide the critical support necessary, consequently equipping research teams to effectively confront and resolve these complex developmental barriers. We enable the significant acceleration of drug discovery timelines. Moreover, leveraging sophisticated proprietary virotherapy platforms combined with profound expertise in precision oncolytic virus engineering facilitates the design and implementation of novel treatment paradigms for these cancers.
How Creative Biolabs Oncolytic Virotherapy Can Assist Your Project
Creative Biolabs provides comprehensive oncolytic virotherapy development services to support your research in hematologic malignancies. We offer tailored solutions to meet your specific project needs, from initial design to final validation.
Workflow
Estimated Timeframe:
Pre-requirement communication: 1-2 weeks
Design and construction of oncolytic viruses: 3-4 weeks
Mass production of oncolytic viruses: 2-3 weeks
Function and properties of oncolytic viruses in vivo and in vitro: 3-4 weeks
Results analysis and test report: 1-2 weeks
Product delivery and shipping: 2-3 weeks
Why Choose Us?
We offer several key advantages:
- Expertise and Experience: Our team comprises experienced scientists with extensive knowledge in virology, immunology, and oncology.
- Cutting-Edge Technology: We utilize state-of-the-art facilities and advanced technologies to ensure high-quality results.
- Customized Solutions: We tailor our services to meet your specific research needs and project goals.
- Comprehensive Services: We provide a full range of services, from initial design to final validation, streamlining your research process.
- Published Data: Our work is supported by published data demonstrating the efficacy and safety of oncolytic virotherapy.
Case Study
The employment of genetically-modified oncolytic viruses in widely used in vivo murine models and in vitro cell-line models of hematological malignancies has demonstrated a substantial increase in tumor lysis rates. Data culled from several published research articles offer valuable insights into its promising prospects within the context of hematological malignancy treatment.
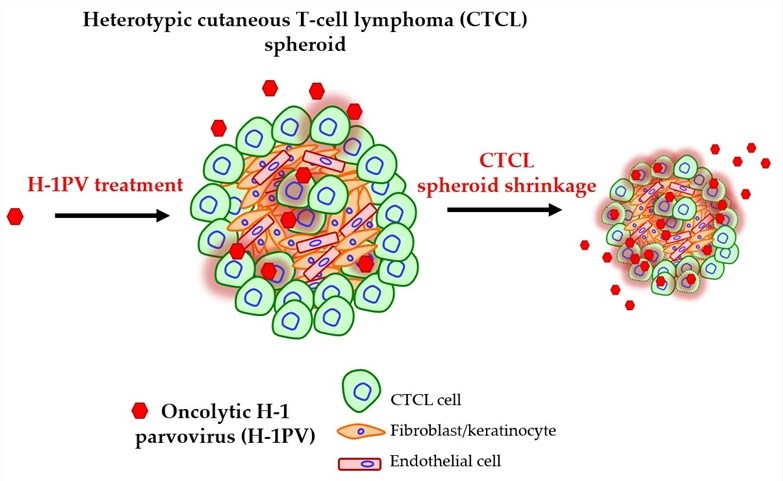 Fig.1 Mechanism of oncolytic virus H-1PV in the treatment of cutaneous T-cell lymphoma.1
Fig.1 Mechanism of oncolytic virus H-1PV in the treatment of cutaneous T-cell lymphoma.1
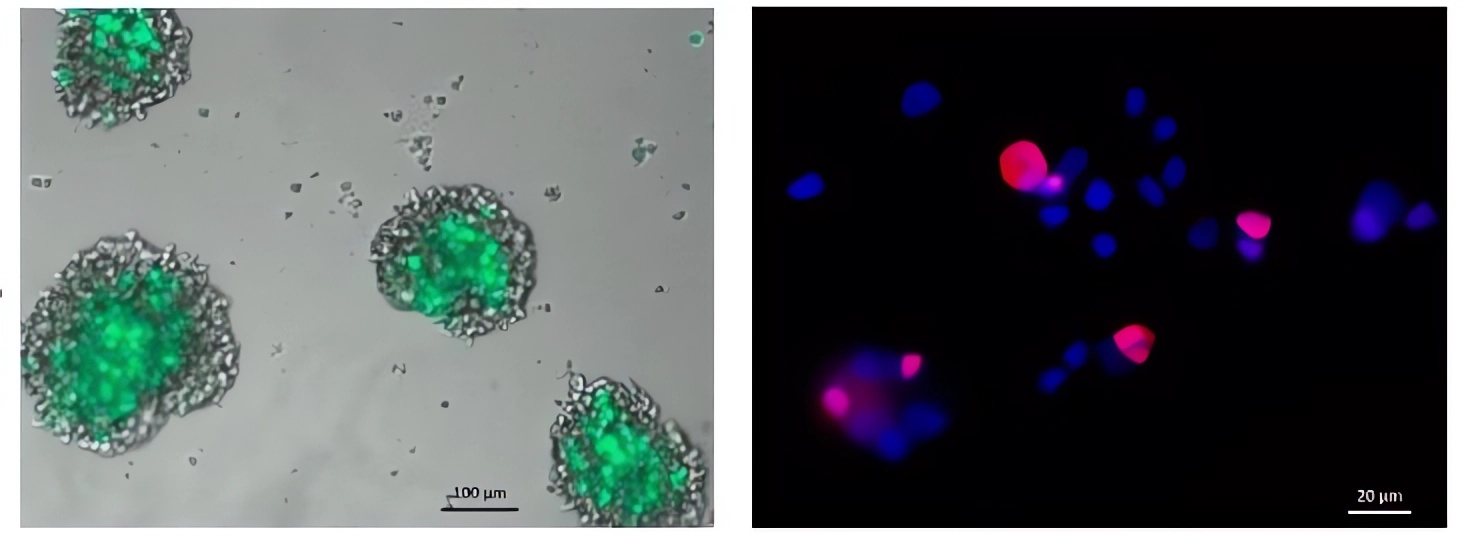 Fig.2 The oncolytic virus successfully infects the tumor cells.1
Fig.2 The oncolytic virus successfully infects the tumor cells.1
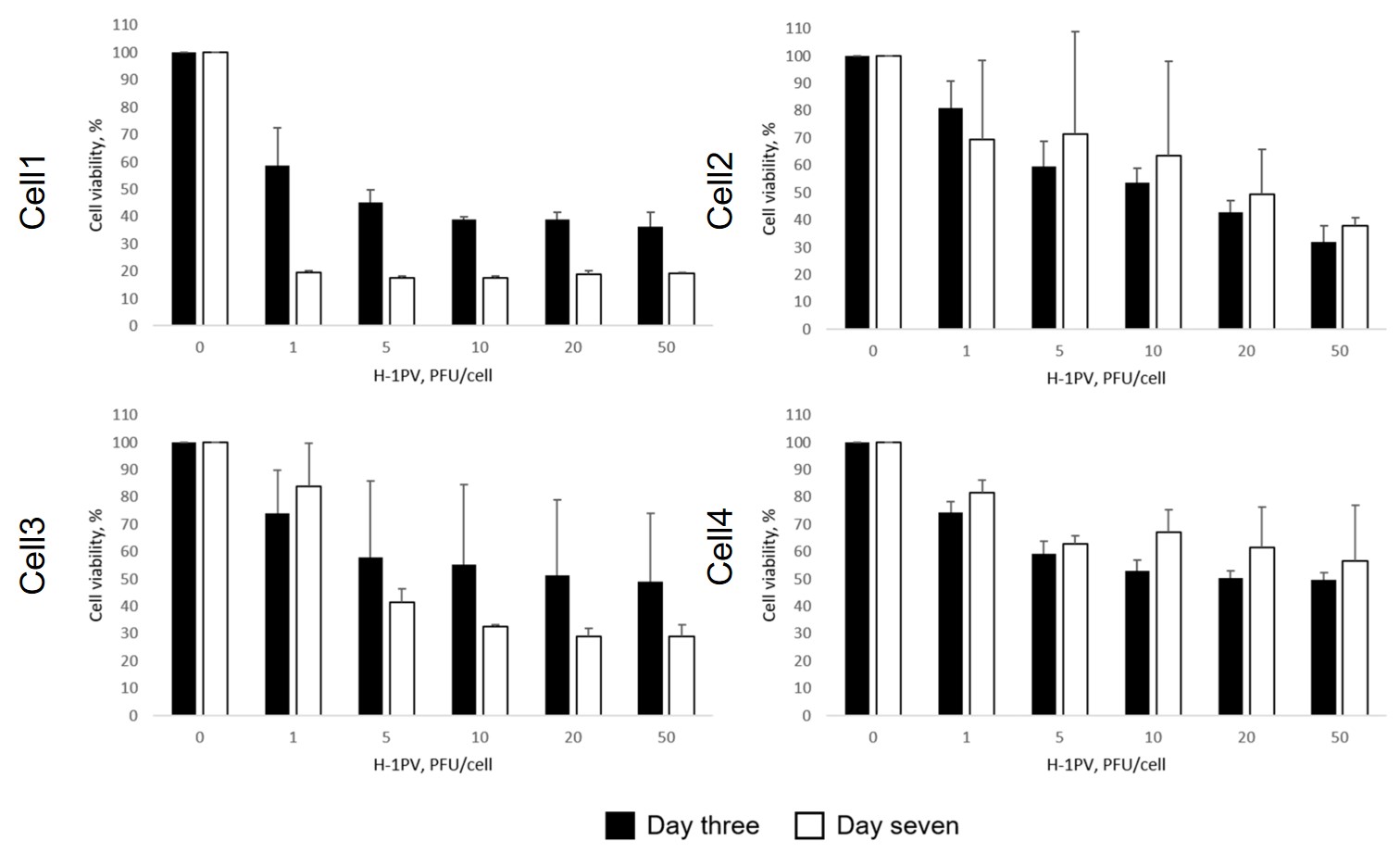 Fig.3 Oncolytic viruses reduce tumor cell activity.1
Fig.3 Oncolytic viruses reduce tumor cell activity.1
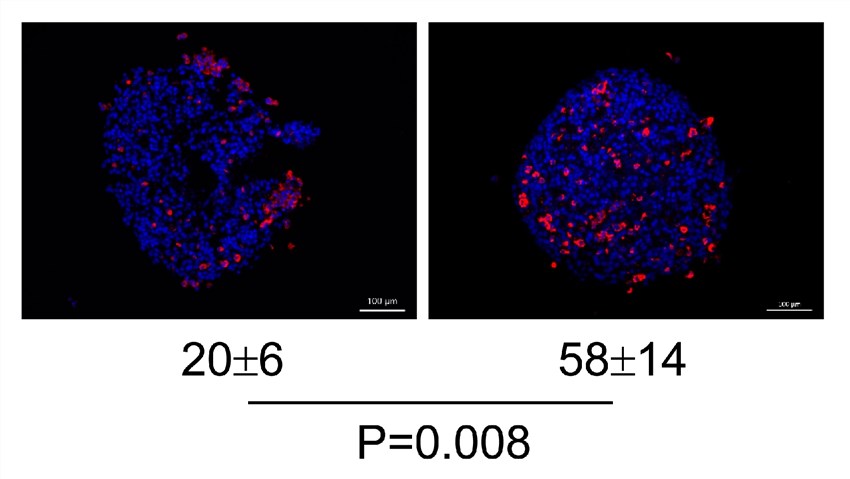 Fig.4 Oncolytic virus increases CD8+ T cell infiltration (red).1
Fig.4 Oncolytic virus increases CD8+ T cell infiltration (red).1
Customer Reviews
-
"The expertise and support provided by Creative Biolabs were crucial in facilitating our in vivo studies. Their oncolytic viruses showed remarkable efficacy in our hematological malignancy models."
-Dr. Garcia, Cancer Center.
-
"Creative Biolabs tailored approach and efficient workflow have accelerated our drug development timeline. We were particularly impressed with the quality of their data and reporting."
-Dr. Kim, Biotech Company.
Highlight
Our key strengths include:
- One-stop service: From initial study design and oncolytic virus selection to preclinical in vitro and in vivo studies, data analysis, and reporting, we provide a complete end-to-end solution.
- Efficient process development: We optimize both upstream (virus production) and downstream (virus purification) processes to maximize yield and purity.
- Large-scale production capability: Our state-of-the-art facilities feature industrial-scale fermentation tanks with capacities from 4,000L to 12,000L, enabling us to support projects requiring large volumes of oncolytic viruses.
- Quality assurance: We adhere to a well-established quality system, incorporating Quality-by-Design (QbD) principles and Process Analytical Technology (PAT) to ensure product consistency and quality.
- GMP-compliant production: Our fermentation processes are conducted under GMP-certified conditions, ensuring the suitability of our products for clinical applications.
- Codon Optimization and Expression Enhancement: We optimize the codon usage of genes to facilitate expression in selected microorganisms, maximizing protein production.
- Safety and Compliance: We follow Hazard Analysis Critical Control Point (HACCP) principles and Good Manufacturing Practices (GMP) to ensure the safety and quality of our processes and products.
- High-Standard Quality Control: We employ high-standard quality control tools to accurately quantify and evaluate the quality of your oncolytic virus products.
At Creative Biolabs, we know that each project is different. That’s why we provide personalized services to fit your particular research, development, and production requirements. Reach out to us today to talk about how we can help you move your oncolytic virotherapy research forward.
Introduction of Oncolytic Virus Therapy for Hematologic Malignancies
Hematologic Malignancies often affect people who are 60 years old or older. This includes lymphomas, leukemias, multiple myeloma (MM), and myelodysplastic syndromes (MDS). Because these cancers are so risky, getting a hematopoietic stem cell transplant (HCT) is seen as a good way to treat them. However, in the context of autologous HCT, recurrence due to cancer cell contamination within the autograft persists as a major hurdle. Additionally, efforts have been made to explore ex vivo modifications of the autograft to eliminate cancer cells using chemotherapies and toxins. So far, an optimal ex vivo purging agent should possess several characteristics, such as precisely targeting the contaminating cancer cells while protecting normal stem and progenitor cells and being applicable rapidly without causing harm to the recipient. OV is a fitting candidate that satisfies these requirements. Boasting top-notch experts and extensive experience in immunotherapy, Creative Biolabs has built an advanced OncoVirapy™ platform.
Utilizing our OncoVirapy™ platform, our researchers have the expertise to formulate customized oncolytic virotherapy regimens for hematological malignancies. Additionally, we offer OV construction and validation services to clients worldwide.
 Distributed under CC VY-SA 3.0, from Wiki, without modification.
Distributed under CC VY-SA 3.0, from Wiki, without modification.
 Distributed under Public domain, from Wiki, without modification.
Distributed under Public domain, from Wiki, without modification.
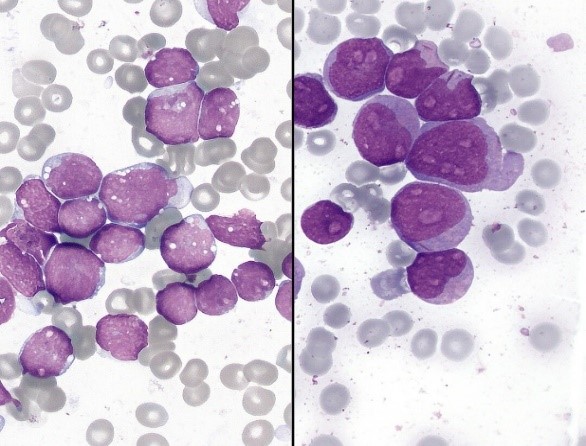
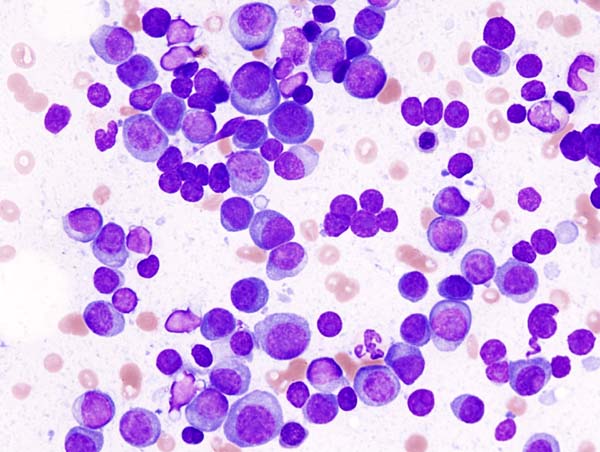
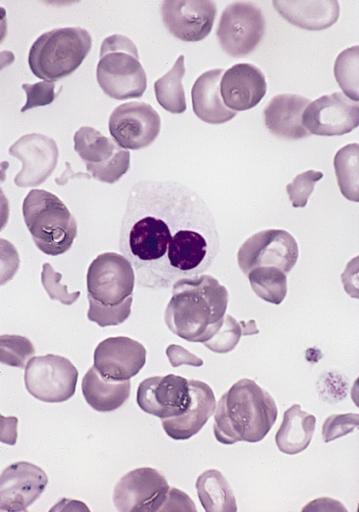
Fig.5 Smears of common hematological neoplasms (Left: Multiple myeloma-May-Grunwald-Giemsa. Middle: leukemia-Wright-Giemsa stain. Right: Myelodysplastic syndrome-Wright-Giemsa stain).
Oncolytic Virus Therapy for Hematologic Malignancies
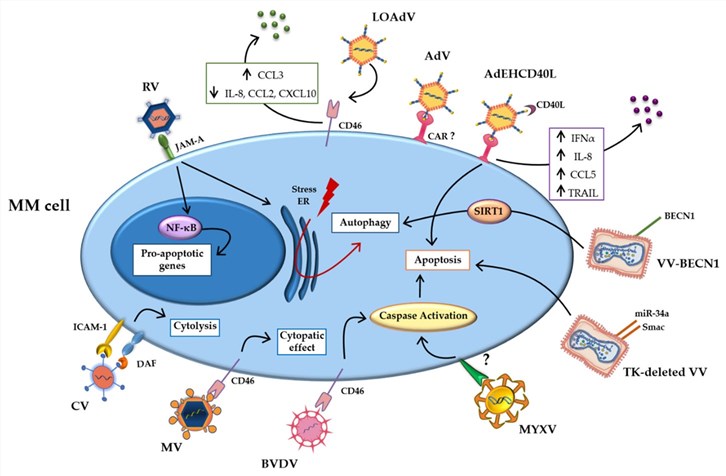 Fig.6 Different classes of oncolytic viruses treat MM.2
Fig.6 Different classes of oncolytic viruses treat MM.2
Oncolytic virus therapy employs specific viruses to target and destroy hematologic malignancy cells. Here are some examples of oncolytic virus therapies being explored for hematologic malignancies:
- ReV
An unmodified Dearing strain type III reovirus (RV) is used to treat the relapsed MM.
- T-VEC and Potential Hematologic Applications
T-01, a designated third-generation oncolytic variant of HSV-1, effectively promotes cell death pathways within MM cells.
- Measles Virus Clinical Trials in MM
For instance, pivotal clinical studies conducted at the Mayo Clinic have evaluated measles virus vector-based approaches specifically within populations presenting with relapsed or refractory MM, initial reports suggest a favorable safety profile alongside encouraging anti-myeloma biological activity.
- Application of recombinant oncolytic vaccinia virus
A modified oncolytic vaccinia virus, with the TK gene removed and made to produce microRNA let-7a, shows it can work well and is safe for treating blood cancers.
FAQ
Q: Which kinds of blood cancers are amenable to treatment with oncolytic virotherapy?
A: Oncolytic virotherapy is applicable for treating leukemias, lymphomas, multiple myeloma, and the like.
Q: How do oncolytic viruses play a role in the special hematological tumors microenvironment?
A: Key detrimental factors include inhibitory cytokines originating from bone marrow stromal cells. Additionally, the dense extracellular matrix further hinders viral activity and spread. Genetic modification techniques can be used to enhance the safety and the validity of OVs
Q: Are you able to assist with the preclinical and clinical advancement of oncolytic virotherapy?
A: Creative Biolabs provides an all-encompassing platform for oncolytic virotherapy development, aimed at promoting scientific research in this domain. We're eager to aid customers in resolving issues that arise during pre-clinical trials.
References
- Angelova, Assia, et al. "H-1 Parvovirus-Induced Oncolysis and Tumor Microenvironment Immune Modulation in a Novel Heterotypic Spheroid Model of Cutaneous T-Cell Lymphoma." Cancers 16.15 (2024): 2711. DOI: 10.3390/cancers16152711. Distributed under Open Access license CC BY 4.0, with re-format the picture.
- Marchica, Valentina, et al. "Oncolytic virotherapy and microenvironment in multiple myeloma." International Journal of Molecular Sciences 22.5 (2021): 2259. DOI: 10.3390/ijms22052259. Distributed under Open Access license CC BY 4.0, without modification.