Complement-Related Diseases Classification of Complement Deficiencies Targeting Complement Pathways Related Products Resources
Complement-Related Diseases
The complement system historically has been recognized as a component of immune defense against foreign invaders
(e.g. bacteria, viruses, and fungi) by opsonization and cytolysis. Recent studies showed that its versatile
functions extend far beyond the elimination of microbes. The complement has verified as a surveillance system to
discriminate healthy and altered host cells (e.g. apoptotic and necrotic cells and their fragments), because of the
capacity to recognize and dispel the damaged "self" components.
By sending "danger" signals and orchestrating immune responses, complement system contributes substantially to
homeostasis, but it may damage healthy host cells and tissues if not properly controlled. With a major functional
linkage with other systems, including coagulation, adaptive immunity, Fc receptors, TLRs, and noninflammatory
disposal mechanisms, the intricate complement network requires rigorous regulation to maintain appropriate
homeostasis. Excessive or insufficient activation will break the delicate balance between complement activation and
regulation, leading a variety of disease, including:
Classification of Complement Deficiencies
The complement system consists of more than 30 proteins and regulators that play a key role in innate immunity by
mediating processes such as conditioning, pathogen lysis, and inflammation. Nearly 50% of all complement proteins serve as regulators or inhibitors in the amplification
cascade, so any absence or suboptimal function of the proteins gives rise to severe clinical disease.
Based on the pathway of complement activation, complement deficiencies can broadly be categorized into the following
three groups.
|
By Pathway
|
Type of Complement Deficiency
|
|
Classical Pathway Deficiency
|
C1q deficiency is associated with an increased susceptibility to systemic lupus erythematosus (SLE) or SLE-like syndrome with fever, rash,
arthritis, and glomerulonephritis. The investigation revealed that C1q deficiency contributes to the
encapsulated bacteria induced infection and high morbidity in childhood from recurrent bacterial/viral
infections.
|
|
C2 deficiency is the most common homozygous complement deficiency, which also relates to SLE-like
illness, infections, rheumatic disorders, and atherosclerosis.
|
|
C3 deficiency may be a reason for recurrent pyogenic infections and membrano-proliferative glomerulonephritis.
|
|
Alternative Pathway Deficiency
|
Patients with properdin deficiency present hypersensitivity to Neisseria infections (meningococcus and
gonococcus) which can be fulminant and life-threatening.
|
|
Factor D deficiency shows an insufficiency of alternative pathway hemolytic activity, and it can greatly
reduce opsonization of encapsulated bacteria, Streptococcus pneumoniae. While there is no evidence for
the association between factor D deficiency and autoimmunity.
|
|
Lectin Pathway Deficiency
|
Children with MBL deficiency are high-risk group for infection, especially in the period that the
passive immunity (acquired from maternal IgG) has been lost, while the protective antibody has not
formed yet. MBL deficiency has a very high prevalence with increased susceptibility and clinical
severity of fungal, protozoal, and viral infections as well as community-acquired pneumonia and
Neisseria meningitis.
|
Complement deficiencies can broadly be broadly categorized as primary or secondary based on their origin and
underlying mechanisms.
-
Primary complement deficiencies arise from genetic mutations that disrupt the synthesis, structure, or function
of complement components or regulatory proteins. These
deficiencies are often hereditary and can lead to recurrent infections,
immune complex diseases, or autoimmune conditions.
-
Secondary complement deficiencies occur as a result of acquired conditions or diseases that lead to the
depletion, consumption, or functional inhibition of complement components. These deficiencies are not caused by
genetic mutations but by pathological processes that impact complement system functionality.
|
|
Primary Deficiencies
|
Secondary Deficiencies
|
|
Origin
|
Genetic mutations
|
Acquired conditions or diseases
|
|
Mechanism
|
Structural or functional defects
|
Dysregulation or depletion
|
|
Examples
|
C3 deficiency, Properdin deficiency
|
SLE, infections, malignancies
|
|
Clinical Onset
|
Early in life (often hereditary)
|
Varied, depending on the underlying cause
|
|
Management
|
Enzyme replacement, prophylactic antibiotics
|
Treat underlying disease, complement modulation
|
|
Research Tools
|
|
Clinical studies reveal that excessive activation of complement system causes massive accumulation of complement
components in the serum of rheumatoid arthritis (RA). In addition, age-related macular degeneration (AMD) and coagulation disorders
are related to the excessive accumulation of complement.
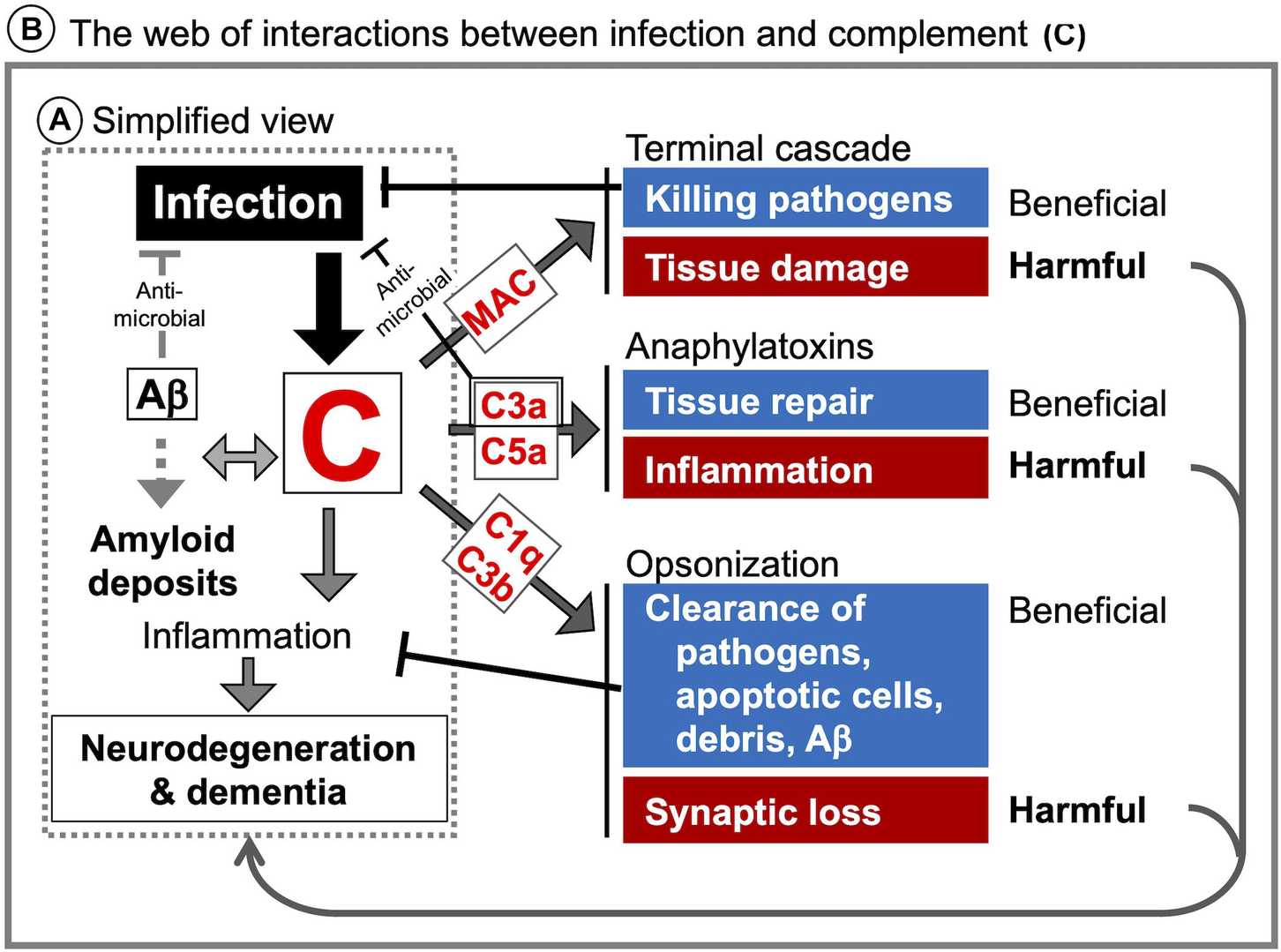
Fig. 1 Illustration of complement system related CNS diseases.1, 2
Targeting Complement Pathways for Drug Discovery
By targeting complement pathways, developing biologics to modulate complement activity, and harnessing the power of
gene therapy, researchers and biopharmaceutical companies are opening new avenues for treating these complex
diseases. At Creative Biolabs, we are dedicated to complement systems research, providing cutting-edge solutions to
advance complement therapies.
Based on our experienced team of experts and well-established complement platform, Creative Biolabs provides a great
variety of therapeutic antibodies, inhibitors, soluble complement regulators, as well as customized services to help
our customers develop novel drugs for complement-related diseases.
-
Alternative therapies - This therapy involves the use of purified or recombinant complement proteins to restore
function, such as C1-INH concentrates, plasma-derived C3 or C5 proteins.
-
Complement inhibitors - Targeted inhibitors are
effective in cases of complement overactivation or dysregulation. The future of complement component inhibitor
development promises to provide more targeted and effective treatments for a wide range of diseases.
-
Small molecule modulators - Small molecules targeting complement activation pathways are being developed to
modulate complement activity in a tissue-specific or pathway-selective manner. These approaches are particularly
useful for chronic diseases associated with complement, such as AMD and Alzheimer's disease.
-
Gene therapy - Gene therapy holds the promise of addressing genetic defects in complement components.
Related Products
Our comprehensive complement platform offers a great number of complement-related products in a rapid and
cost-effective manner. If you are interested, please feel free to contact us for more
details.
|
Cat.No
|
Product Name
|
Purity
|
Applications
|
|
CTA-756
|
Mouse Anti-Human Cl Inhibitor Mono-
clonal Antibody
|
≥95%
|
ELISA; WB; IP
|
|
CTA-526
|
Mouse Anti-Human Complement C1q
Monoclonal Antibody
|
≥95%
|
WB; ELISA
|
|
CTA-009
|
Mouse Anti-Human Complement
C3a/C3a desArg Monoclonal Antibody
|
≥95%
|
FC; IHC; Neut
|
|
CTA-004
|
Mouse Anti-Human Complement C3
Monoconal Antibody
|
≥95%
|
ELISA; WB
|
|
CTA-093
|
Mouse Anti-Rat Complement C3b
Monoclonal Antibody
|
≥95%
|
WB; IA
|
|
CTA-118
|
Mouse Anti-Human Complement C5a
Monoclonal Antibody
|
≥95%
|
WB; ELISA; Neut
|
|
CTA-053
|
Mouse Anti-Human Complement C9
Monoclonal Antibody
|
≥95%
|
WB; ELISA; ICC/IF;
IHC; ICC/IF
|
|
CTA-551
|
Mouse Anti-Rat C5b-9 complex Mono-
clonal Antibody
|
≥95%
|
WB; ELISA; IHC; FC; I
|
|
CTP-461
|
Native Human Complement Clg
Protein
|
>95% by
SDS-PAGE
|
ELISA, Functional
Assays
|
|
CTP-033
|
Recombinant Human
Complement 1 Inhibitor
|
>95% by
SDS-PAGE
|
Activity Assays
|
|
CTP-460
|
Native Human
Complement C1 Complex Protein
|
>95% by
SDS-PAGE
|
ELISA, Functional
Assays
|
|
CTP-505
|
Native Human Complement C3
Protein
|
>95% by
SDS-PAGE
|
ELISA, Functional
Assays
|
|
CTP-044
|
Recombinant Human
Complement C3a Protein
|
>95% by
SDS-PAGE
|
Bioactivity Assays
|
|
CTP-262
|
Native Human
Complement C5 Protein
|
>95% by
SDS-PAGE
|
ELISA, Functional
Assays
|
|
CTP-268
|
Native Human
Complement C5a Protein
|
>95% by
SDS-PAGE
|
ELISA, Functional
Assays
|
|
CTP-293
|
Native Human Complement
SC5b-9 Complex Protein
|
>95% by
SDS-PAGE
|
ELISA, Functional
Assays
|
|
CTI-001
|
SB290157 (Trifluoroacetate)
|
95%
|
Inhibit ~90% complement activity in a hemolytic assay
at Conc. 0.77mM
|
|
CTI-002
|
PMX-53
|
98%
|
C5a receptor antagonist, inhibits C3aR with the
IC50 of 20 nM.
|
|
CTI-005
|
W-54011
|
98%
|
C5a receptor antagonist, it inhibits C5aR with the Ki
value of 2.2 nM.
|
|
CTI-006
|
Complement C5-lN-1
|
98%
|
Potent C5 inhibitor, it blocks zymosan-induced the
membrane-attack complex (MAC) deposition in 50%
human whole blood with an IC50 of 0.77 μM.
|
|
CT1-009
|
FD-IN-1
|
98%
|
Pfactor D antagonist, it inhibits factor D with the
IC50 of 12 nM.
|
|
CTI-010
|
Compstatin
|
98%
|
Inhibits C3 convertase with the lc50 of 28 uM and the
activation of classical and alternative complement
pathways with the lC50 of 63 and 12 uM, respectively.
|
|
CTI-012
|
ADH-503
|
98%
|
Potent CDllb agonist, it can induce the repolarization
of tumor-associated macrophages.
|
Resources
References
-
Shinjyo, Noriko, Wataru Kagaya, and Marcela Pekna. "Interaction between the complement system and infectious
agents–a potential mechanistic link to neurodegeneration and dementia." Frontiers in Cellular
Neuroscience 15 (2021): 710390.
-
under Open Access license CC BY 4.0, without modification.
For Research Use Only.
Related Sections: