Classification Key Proteins Complement Services
Complement component proteins represent one of the most ancient and critical defense systems in the human body. As a part of innate immunity, the complement system comprises over 30 soluble and membrane-bound proteins that orchestrate a rapid, robust response against pathogens. These proteins are not only vital in pathogen elimination through mechanisms like opsonization, lysis, and chemotaxis, but also serve as critical modulators of adaptive immunity, inflammation, and homeostasis.
Creative Biolabs offers comprehensive complement research services supporting mechanistic studies, biomarker discovery, and drug development targeting individual complement components.
Classification and Activation Pathways of Complement Proteins
The complement system is activated through three pathways:
-
Classical pathway – Triggered by antigen-antibody complexes; relies on C1q, C1r, C1s.
-
Lectin pathway – Initiated by binding of mannose-binding lectin (MBL) or ficolins to carbohydrates on microbial surfaces.
-
Alternative Pathway – Continuously activated at low levels via spontaneous hydrolysis of C3 and amplified on pathogen surfaces.
These three pathways converge at the central molecule C3, whose cleavage into C3a and C3b marks a point of irreversible amplification and downstream effector function. Complement component proteins are generally classified into three categories based on their functional roles in the cascade.
Table 1 Complement protein categories based on their functional roles.
|
Category
|
Examples
|
Functional Role
|
|
Initiator proteins
|
C1q, MBL, ficolins
|
Recognize and bind to pathogens to trigger cascade
|
|
Enzymatic proteins
|
C1r, C1s, MASPs
|
Proteases that cleave downstream complement proteins
|
|
Effector proteins
|
C3, C5b-9
|
Mediate opsonization, inflammation, or direct lysis
|
Key Complement Component Proteins
The complement system consists of a large number of distinct plasma proteins that react with one another to opsonize pathogens and induces a series of inflammatory responses which are helpful to fight infection. There are major complement proteins, C1, C2, C3, C4, C5, C6, C7, C8, C9, factor B, factor D, factor I and factor properdin.
The classical pathway of complement is activated upon binding of the C1 complex. C1 comprises two weakly interacting subunits, C1q and C1r2s2. C1 circulates in a precursor state and C1 activation can be induced by immune complexes or certain nonimmune substances. With C1 binding to an immune complex, the strength of interaction between C1q and C1r2s2 increases. C1 functions are controlled by the serum glycoprotein C1-inhibitor which blocks the enzymatic activities of activated C1.
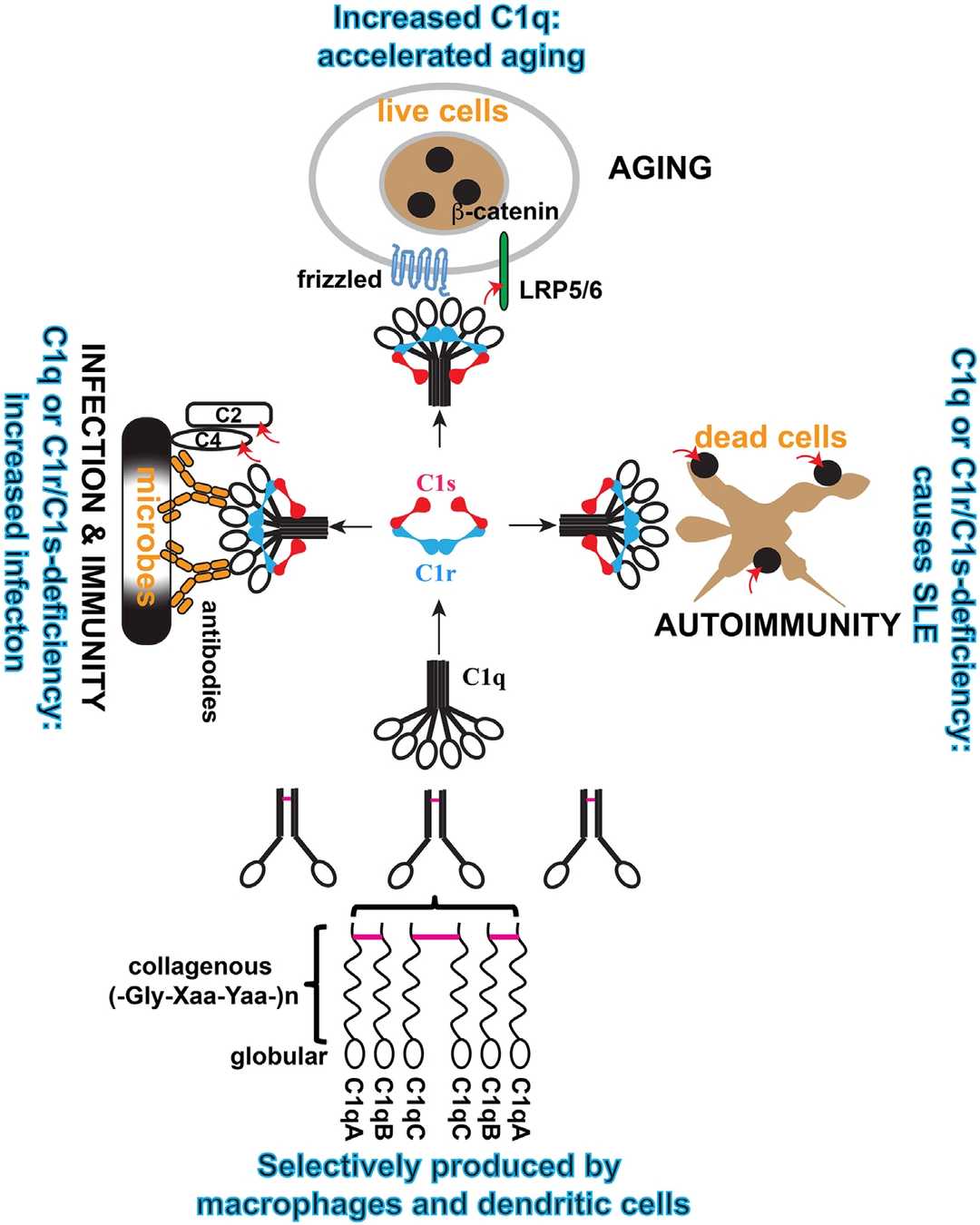 Fig. 1 Structure and assemble of C1.1, 5
Fig. 1 Structure and assemble of C1.1, 5
C2 is a multidomain serine protease that provides catalytic activity to the C3 and C5 convertases of the classical pathway and the lectin pathway of complement. C2 shares homology with the classical serine proteinases, but it is unusual in having a catalytic chain with a much extended N-terminus. Deficiency of C2 has been associated with certain autoimmune diseases.
C3 is a large glycoprotein with a molecular weight of approximately 185 kDa, processed in plasma into two chains: an α-chain (about 115 kDa) and a β-chain (about 70–75 kDa), linked by a disulfide bond. The precursor C3 protein is cleaved during activation, resulting in the fragments C3a and C3b. C3a is a small anaphylatoxin, while C3b is a larger fragment that plays a key role in immune defense.
C4 is encoded by two closely linked and highly polymorphic genes, C4A and C4B, both located in the MHC class III region on chromosome 6. C4 is essential in the activation of the classical and lectin complement pathways. It is also involved, to a lesser extent, in the alternative pathway.
The complement system is activated via three routes, all leading to the activation of C5 and the terminal pathway. C5 is a glycoprotein whose cleavage into bioactive fragments C5a and C5b drives both protective immunity and pathological inflammation. At present, C5 is a validated drug target and an anti-C5 antibody is a therapy for paroxysmal nocturnal hemoglobinuria.
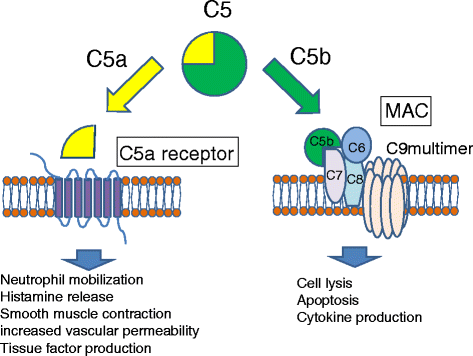 Fig 2 Activation of C5 by C5 convertase leads to the generation of C5a an activities.2, 5
Fig 2 Activation of C5 by C5 convertase leads to the generation of C5a an activities.2, 5
C6 is a part of the lytic membrane attack complex (MAC) formed during complement activation. In C6, the regulatory segment includes four auxiliary domains that stabilize the closed conformation, inhibiting release of membrane-inserting elements. A deficiency of C6 causes enhanced susceptibility to gram-negative bacterial infections or recurrent bacterial infections.
Complement component C7 is a single-chain glycoprotein consisting of a single domain (MACPF) and eight cysteine-rich modules homologous. C7 plays an integral role in the assembly of this complex within target cell membranes. A deficiency of C7 leads to the dysfunction of MAC.
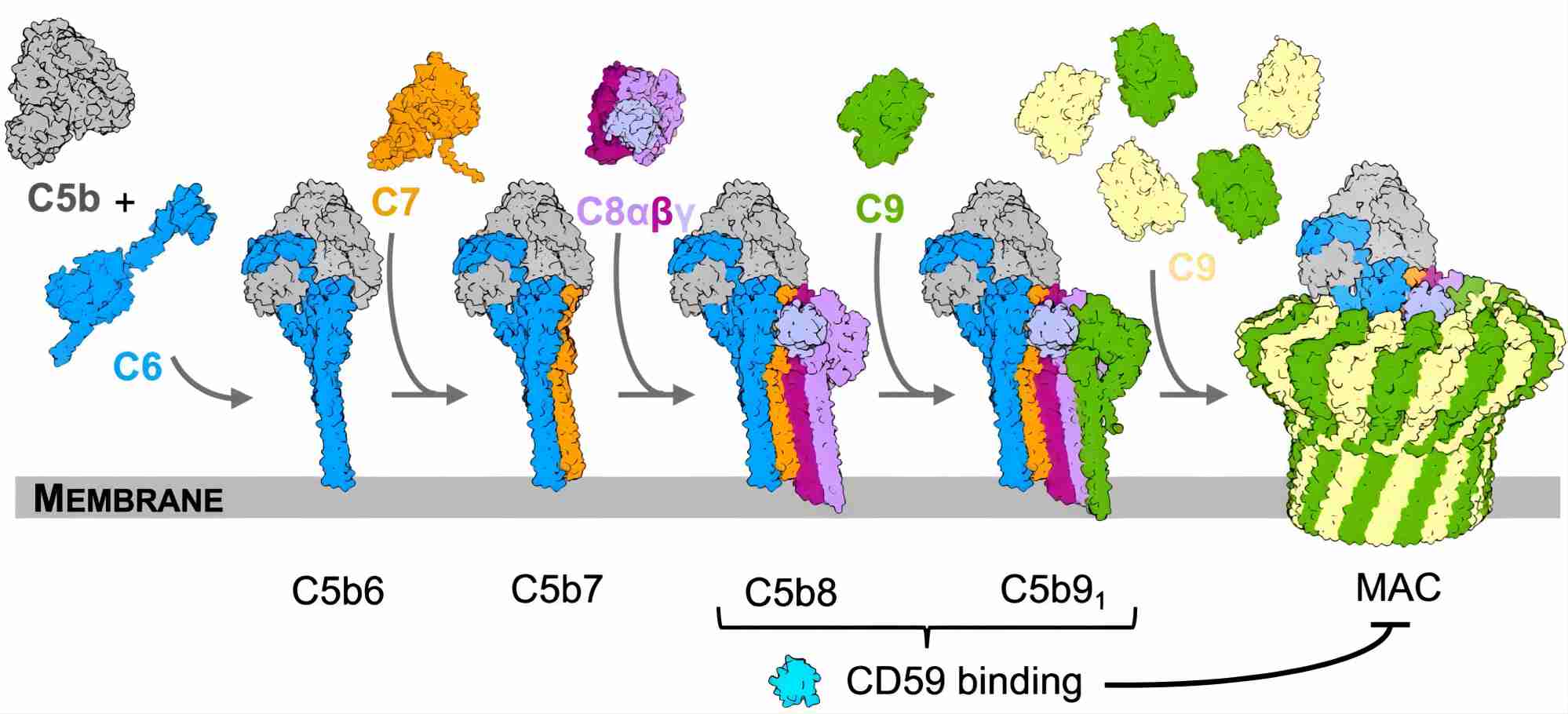 Fig. 3 Complement C7 participates in the MAC assembly.3, 5
Fig. 3 Complement C7 participates in the MAC assembly.3, 5
Complement component C8 is composed of three subunits (alpha, beta and gamma). Together with C5, C6, C7 and C9, C8 assembles on bacterial membranes to form the lethal pore-like MAC. Deficiency of C8 is a very rare primary immunodeficiency, associated with invasive, recurrent infections mainly caused by Neisseria species.
Complement component C9 is the last protein that binds to the assembling MAC of complement, completing the sequence of events that leads to the destruction of target membranes. During MAC assembly, up to 18 molecules of C9 can bind to each C5b-8 complex, forming stable ion pores or channels in membranes and leading to lysis and death of the target cell.
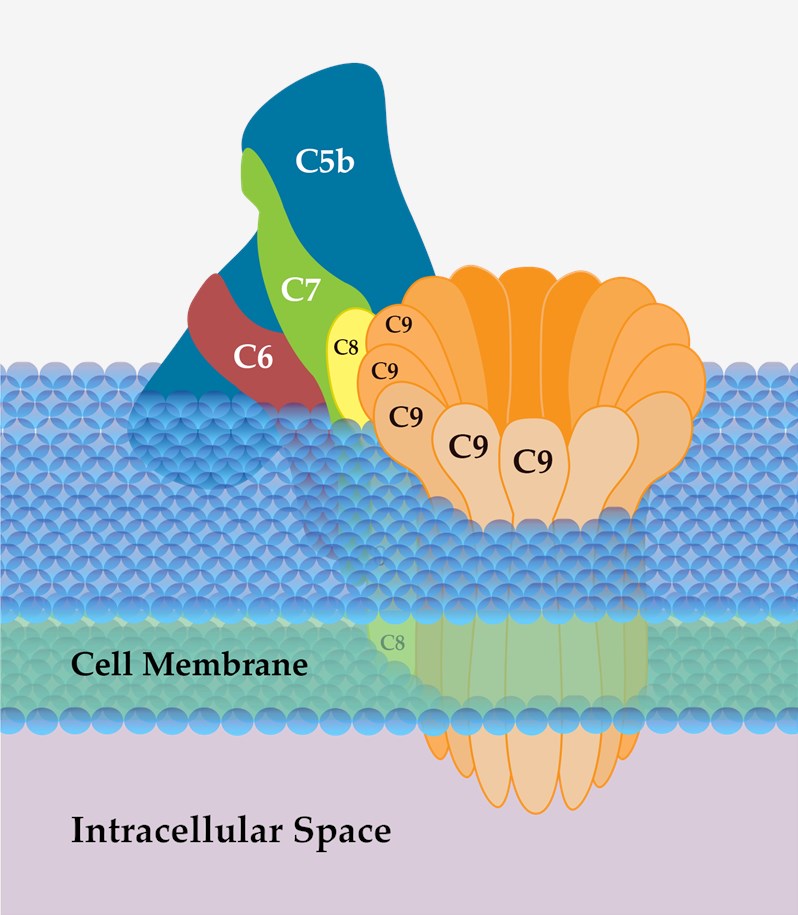 Fig 4 Schematic diagram of MAC.4
Fig 4 Schematic diagram of MAC.4
Complement component factor B, a single-chain, 93 kDa pcolypeptide, is a component of the alternative pathway of complement. Factor B circulates as an inactive proenzyme and only becomes activated after cleavage by factor D. It is generated as a single-chain protein, and cleavage by factor D generates two peptide fragments, Ba (33 kDa) and Bb (60 kDa). When activated, it provides the catalytic activity of the alternative pathway C3 and C5 convertases.
Complement component factor D is a serine protease consisting of a single polypeptide of 228 amino acids. The function of factor D is to cleave its unique substrate to generate the C3 convertases C3(Hc2O)Bb and C3bBb. Factor D participates in the amplification loop which contributes significantly to responses elicited by the classical pathway and the lectin pathway.
Complement component factor properdin is a key positive regulator of the alternative pathway by significantly increasing the half-life of the C3 and C5 convertases. Properdin is also an initiator of the alternative pathway. The properdin gene is located on the X chromosome and a deficiency of factor properdin leads to impaired alternative pathway function.
Complement factor I, also known as C3b/C4b inactivator, is a crucial serine protease in the complement system. It is encoded by the CFI gene on chromosome 4 and primarily produced in the liver, though also synthesized in monocytes, fibroblasts, keratinocytes, and endothelial cells. CFI is a key inhibitor of the complement system, controlling both the classical and alternative pathways.
Complement Services at Creative Biolabs
Creative Biolabs offers a broad range of tools for complement system studies, including:
Complement component proteins are no longer viewed merely as innate defense molecules—they are now key players in immune regulation, disease progression, and therapeutic development. Understanding their nuanced roles in health and disease is critical for unlocking new diagnostic and treatment strategies.
At Creative Biolabs, we are proud to support global researchers with tailored solutions for complement studies, from basic research to therapeutic development. Contact us to elevate your complement research with expert services and cutting-edge platforms.
References
-
Lu, Jinhua, and Uday Kishore. "C1 complex: an adaptable proteolytic module for complement and non-complement functions." Frontiers in immunology 8 (2017): 592. https://doi.org/10.3389/fimmu.2017.00592
-
Horiuchi, Takahiko, and Hiroshi Tsukamoto. "Complement-targeted therapy: development of C5-and C5a-targeted inhibition." Inflammation and regeneration 36.1 (2016): 1-5. https://doi.org/10.1186/s41232-016-0013-6
-
Bubeck, D., et al. "Structural basis for membrane attack complex inhibition by CD59." (2023). https://doi.org/10.1038/s41467-023-36441-z
-
From Wikipedia: By SLiva2016 - Own work, CC BY-SA 3.0 https://commons.wikimedia.org/wiki/File:Membrane_Attack_Complex_(Terminal_Complement_Complex_C5b-9).png
-
under Open Access license CC BY 4.0, without modification
For Research Use Only.
Related Sections:

 Fig. 1 Structure and assemble of C1.1, 5
Fig. 1 Structure and assemble of C1.1, 5
 Fig 2 Activation of C5 by C5 convertase leads to the generation of C5a an activities.2, 5
Fig 2 Activation of C5 by C5 convertase leads to the generation of C5a an activities.2, 5
 Fig. 3 Complement C7 participates in the MAC assembly.3, 5
Fig. 3 Complement C7 participates in the MAC assembly.3, 5
 Fig 4 Schematic diagram of MAC.4
Fig 4 Schematic diagram of MAC.4