Immunopathology of SLE Mechanisms of SLE Treatment of SLE Related Products Hot Services Q&A Resources
While the immune system protects health through the destruction of pathogens and bacteria, it mistakenly attacks the body's own cells in autoimmune diseases because of its inability to distinguish them from foreign invaders. Systemic lupus erythematosus (SLE) stands as a significant autoimmune disorder needing novel treatments to achieve effective management. Creative Biolabs has expert scientists in complement therapeutic research to offer a comprehensive range of services that help clients conquer drug development challenges for SLE-like conditions.
Immunopathology of SLE
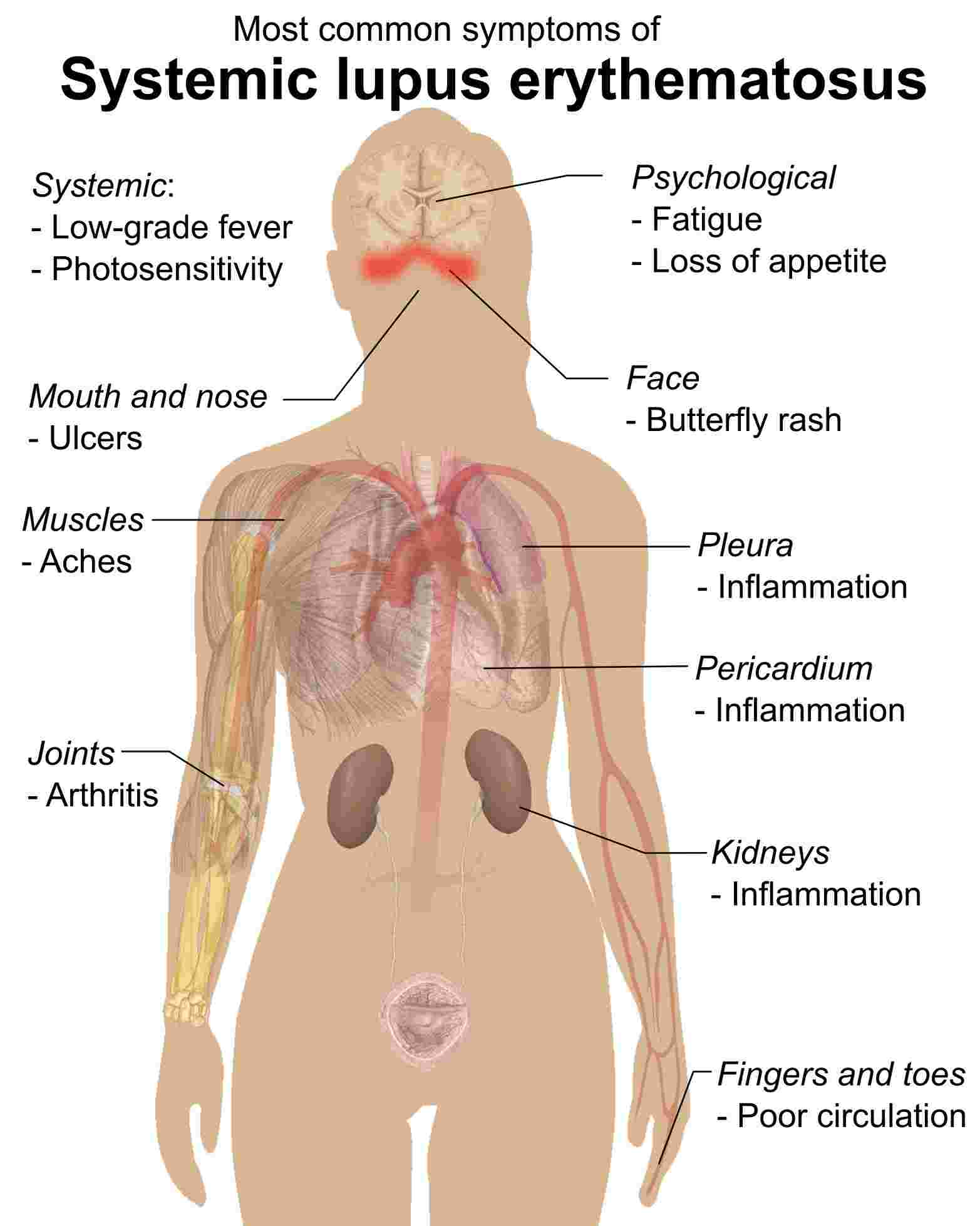 Distributed under the public domain, from Wiki, without modification. Fig. 1 The illustration of systemic lupus erythematosus.1
Distributed under the public domain, from Wiki, without modification. Fig. 1 The illustration of systemic lupus erythematosus.1
The precise cause of SLE remains unclear, but several contributing factors have been identified:
Genetic Factors
Individuals who lack proper function in complement system components such as C1q, C2, or C4 face higher chances of developing SLE. Patients who lack C1q experience autoantibody production and lupus-like symptoms due to defective elimination of apoptotic cells, which increases immune system antigen exposure. Genome-wide association studies have discovered 28 more loci that increase disease susceptibility. The strongest disease risk loci identified are ITGAM, FcγR, PRDM1-ATG5, and TNFAIP3 that present opportunities for new therapeutic approaches.
Environmental Factors
SLE researchers have studied ultraviolet radiation most thoroughly as its primary environmental trigger. Patients experience worsening skin symptoms from exposure to ultraviolet A2 rays as well as ultraviolet B rays. The impact of ultraviolet B radiation on cellular apoptosis operates in a dose-dependent manner while showing concurrent relationships with the liberation of lupus autoantigens and cytokine secretion. A variety of environmental triggers can provoke SLE through specific medications and viral infections, along with physical or emotional stress and trauma.
Sex and Hormones
The female population experiences systemic lupus erythematosus at a much higher rate than males, with a ratio of 9:1. Young females face increased risks, while women in general show higher rates of the condition. Despite lacking conclusive evidence, scientists suspect that estrogen contributes significantly to this gender-specific bias. The symptoms of SLE become more severe in female patients during pregnancy and menstrual cycles. The evidence indicates that estrogen may contribute to SLE development.
Molecular Mechanism of SLE
In SLE, the molecular mechanisms underlying complement-mediated tissue damage are multifaceted and significantly contribute to the disease's diverse clinical manifestations.
Table 1 Complement proteins in SLE.
|
Molecular
|
Function
|
|
C1q
|
Initiates the classical complement pathway when it binds to immune complexes formed by autoantibodies (e.g., anti-dsDNA) and self-antigens in SLE. Its deficiency is strongly linked to increased SLE risk due to impaired immune complex clearance.
|
|
C2&C4
|
Early components of the classical pathway, whose activation is crucial for the formation of C3 convertase. Deficiency in C2 and C4 is associated with a higher susceptibility to SLE.
|
|
C3
|
A central protein in all complement pathways. SLE causes inflammation and immune complex opsonization through tissue deposition of its activated complement factor C3b. C3a and C5a, resulting from C3b cleavage and function as powerful anaphylatoxins.
|
|
C3a, C5, C5a
|
Anaphylatoxin C3a and C5a are generated during C3 cleavage, promoting inflammation by recruiting and activating immune cells in SLE-affected tissues. C5 cleavage by C5 convertase (formed through both classical and alternative pathways in SLE) yields C5a and C5b, initiating the formation of the MAC. C5a plays a significant role in SLE pathogenesis by attracting inflammatory cells (e.g., neutrophils, macrophages) to sites of immune complex deposition and stimulating the release of pro-inflammatory mediators, contributing to tissue damage.
|
|
MAC (C5b-9)
|
Can form on host cells, such as endothelial and epithelial cells in the kidney, leading to direct cellular lysis and contributing to organ dysfunction, particularly in lupus nephritis. Its formation is a key effector mechanism of complement-mediated damage in SLE.
|
|
Factor B
|
A key component of the alternative pathway C3 convertase. Its dysregulation can contribute to the amplification of complement activation in SLE.
|
|
Properdin
|
A positive regulator of the alternative pathway, stabilizes the C3 convertase. Its role in SLE pathogenesis is complex and under investigation.
|
|
Factor H
|
A major regulator of the alternative pathway, preventing excessive activation of host cells. Its deficiency or dysfunction can contribute to increased complement activation in SLE.
|
|
DAF (CD55)
|
A complement regulatory protein that inhibits the formation of both classical and alternative pathway C3 convertases on host cell surfaces, protecting them from complement-mediated damage in SLE. Reduced expression or function can exacerbate tissue injury.
|
|
CD59
|
A membrane-bound protein that inhibits the formation of the MAC on host cell surfaces, preventing lysis. Its deficiency or impaired function can contribute to increased MAC-mediated damage in SLE.
|
Treatment of SLE
A guaranteed cure for SLE has not yet been discovered. All treatments focus on reducing symptom severity. The treatment approach for SLE changes based on symptom severity and the affected body region. Different treatment options include anti-inflammatory drugs to relieve joint pain and stiffness, steroid creams to treat rashes, antimalarial medications for skin and joint conditions, and disease-modifying drugs or targeted immune system agents for serious cases.
Related Hot Products
Our comprehensive complement platform efficiently and economically provides a wide range of complement-associated products. If you are interested, please feel free to contact us for further information.
Table 2 Featured products.
Related Hot Services
Table 3 Complement test services for SLE-related complement studies.
Creative Biolabs has established advanced Complement Therapeutics Platforms, including an antibody engineering platform, a protease inhibitor platform, and a drug discovery platform, and is equipped to offer a full range of biotherapeutics development services regarding drug discovery and validation for SLE. If you are interested in our services, please contact us for detailed information.
Resources
For Research Use Only.
Related Sections: