Product List Background AChE Aptamer Analysis
Background
Acetylcholinesterase (AChE) is a key enzyme crucial for terminating synaptic transmission by hydrolyzing the neurotransmitter acetylcholine (ACh). It is widely distributed in nerve and muscle tissues throughout the body, primarily the central nervous system and neuromuscular junctions. Structurally, AChE exists in three main isomers: globular, tetrameric, and asymmetric forms. Its primary function involves breaking down ACh into choline and acetate, thereby terminating cholinergic neurotransmission and preventing prolonged muscle contraction or nerve excitation. This enzymatic activity is essential for normal neuromuscular function and cognitive processes in vertebrates.
Its Gene ID: 43, UniProtKB ID: P22303, and OMIM ID: 100740.
Catalytic Mechanism of AChE
AChE exhibits high catalytic activity through its active center, comprising three main regions: the esterification site, anion site, and hydrophobic region. The esterification site contains the catalytic triad of three amino acids and accommodates the acetyl group of ACh, facilitating hydrolysis. The anion site interacts with the positively charged quaternary amine of ACh, aiding substrate orientation. Meanwhile, the hydrophobic region which bands to an esterification or quaternary ammonium group, stabilizes the aromatic moiety of ACh.
Biological Function of AChE
When a neural signal transmits and excites or stimulates a cellular membrane, the ACh is released. Upon release, the ACh acts as a neurotransmitter, transmitting signals across nerve junctions. AChE rapidly hydrolyzes ACh into choline and acetate, terminating its action and ensuring precise signal transmission duration. Structural changes in AChE facilitate its binding to ACh, enhancing catalytic efficiency. AChE’s activity regulates neuromuscular function, cognition, and autonomic processes. This enzymatic process is vital for maintaining neurotransmitter balance, optimizing synaptic communication, and modulating physiological responses throughout the nervous system.
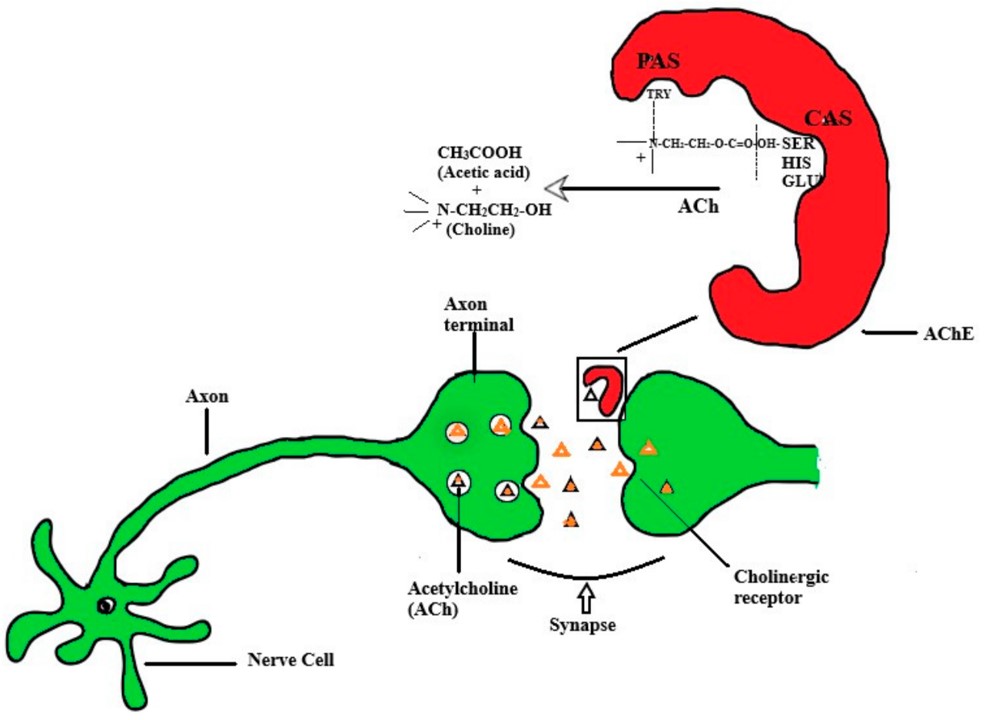 Fig.1 ACh transmission through synapse (cholinergic transmission) and action of AChE on ACh.1, 4
Fig.1 ACh transmission through synapse (cholinergic transmission) and action of AChE on ACh.1, 4
AChE in AD and Other Diseases
In Alzheimer's disease (AD), AChE contributes to pathology by accelerating the breakdown of ACh, exacerbating cognitive decline. AChE’s overexpression in neurons leads to reduced cholinergic signaling, impairing memory and cognition. In other neuromuscular diseases, dysregulation of AChE can disrupt neuromuscular transmission, contributing to symptoms like muscle weakness. Interestingly, in cancer, AChE levels are implicated as potential diagnostic and prognostic signs. Impaired AChE activity has been seen in certain cancers, implying a role in tumor growth and metastasis.
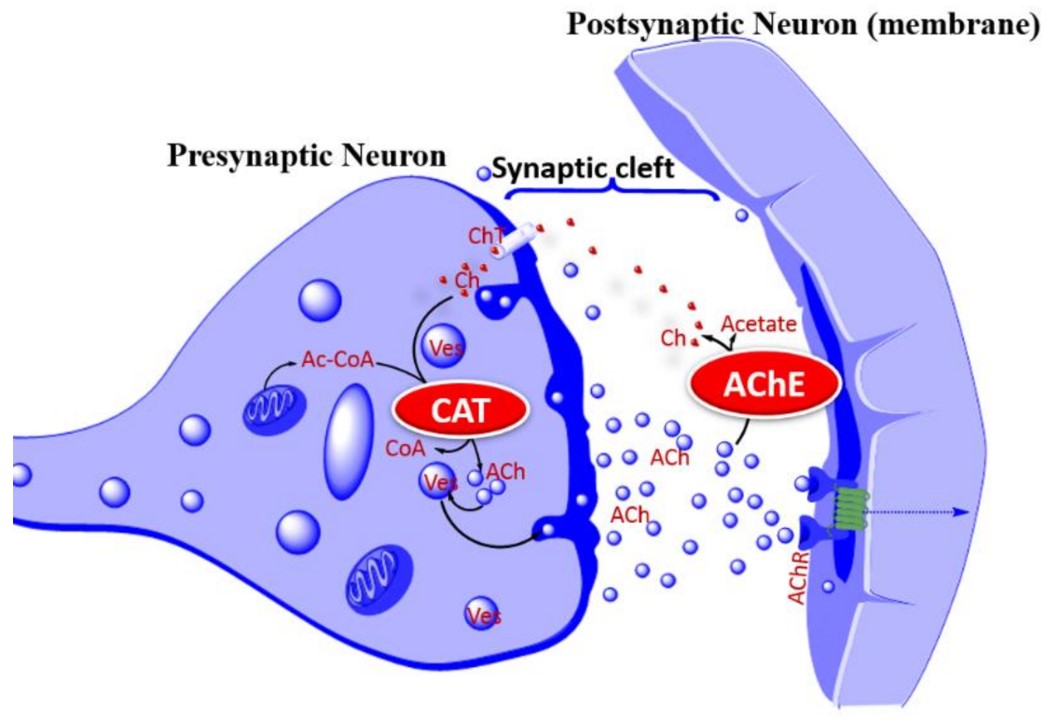 Fig.2 The cholinergic hypothesis of AD.2, 4
Fig.2 The cholinergic hypothesis of AD.2, 4
AChE Inhibitors
AChE inhibitors function by blocking the enzyme AChE. By inhibiting AChE, these drugs increase ACh levels, enhancing cholinergic transmission in the nervous system. AChE inhibitors may be utilized to treat various diseases including AD, myasthenia gravis (MG), and glaucoma. In AD, AChE inhibitors can improve cognitive symptoms by sustaining ACh levels in the brain, temporarily enhancing memory and cognitive function. For MG, AChE inhibitors are the main therapeutic drugs. By inhibiting AChE at the neuromuscular junction, it prolongs ACh's action, improving muscle strength and reducing fatigue. These therapeutic effects highlight AChE inhibitors as vital treatments for conditions characterized by impaired cholinergic neurotransmission, offering symptomatic relief and improving the quality of life for patients.
AChE Aptamer Analysis
Creative Biolabs provides aptamers designed with a specific affinity for the ACHE protein, acclaimed for their precise binding capabilities. These aptamers are crucial in propelling research and innovation in aptamer-based therapeutic and diagnostic strategies. Additionally, we offer functional assays for anti-ACHE aptamers to further meet our client's requirements.
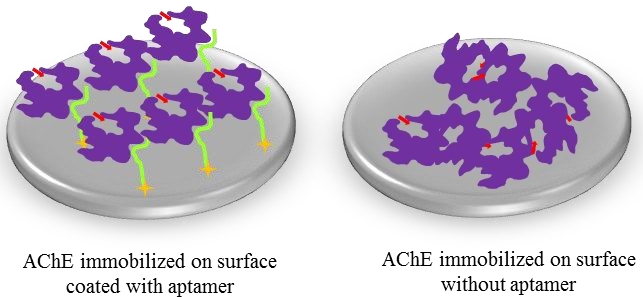 Fig.3 Comparison of AChE site-specific immobilization via aptamer interaction against adsorption onto a polystyrene microtiter plate.3,4
Fig.3 Comparison of AChE site-specific immobilization via aptamer interaction against adsorption onto a polystyrene microtiter plate.3,4
Evaluating AChE aptamer assays entails several phases, starting with the selection of aptamers that specifically bind to anti-AChE antibodies via SELEX technology. To assess binding affinity, the interaction between anti-AChE aptamer and AChE was quantified utilizing the Enzyme-Linked Oligonucleotide Assay (ELONA). In another binding affinity assay, AChE and anti-AChE aptamers were combined with streptavidin-conjugated horseradish peroxidase, and the absorbance was recorded at 450 nm using a multilabel plate reader. The apparent equilibrium dissociation constant (Kd) values were calculated for each aptamer.
Creative Biolabs is dedicated to maintaining high-quality standards and leading innovations in its range of aptamer products. Through the use of cutting-edge technologies and rigorous quality assurance protocols, we offer dependable and effective anti-ACHE aptamers customized to satisfy our clients' specific needs.
References
-
Arya, Ashwani, et al. "Acetylcholinesterase inhibitory potential of various sesquiterpene analogues for Alzheimer’s disease therapy." Biomolecules 11.3 (2021): 350.
-
Habtemariam, Solomon. "Natural products in Alzheimer’s disease therapy: would old therapeutic approaches fix the broken promise of modern medicines?" Molecules 24.8 (2019): 1519.
-
Chumphukam, Orada, Thao T. Le, and Anthony EG Cass. "High efficiency acetylcholinesterase immobilization on DNA aptamer modified surfaces." Molecules 19.4 (2014): 4986-4996.
-
Distributed under Open Access license CC BY 4.0, without modification.


 Datasheet
Datasheet Fig.1 ACh transmission through synapse (cholinergic transmission) and action of AChE on ACh.1, 4
Fig.1 ACh transmission through synapse (cholinergic transmission) and action of AChE on ACh.1, 4
 Fig.2 The cholinergic hypothesis of AD.2, 4
Fig.2 The cholinergic hypothesis of AD.2, 4
 Fig.3 Comparison of AChE site-specific immobilization via aptamer interaction against adsorption onto a polystyrene microtiter plate.3,4
Fig.3 Comparison of AChE site-specific immobilization via aptamer interaction against adsorption onto a polystyrene microtiter plate.3,4
