Product List Background Ficolin-2 Functional Service
Background
Ficolin-2, also known as L-ficolin, is synthesized in the liver and secreted into the bloodstream in humans and it is one of the major pattern recognition molecules of plasma/serum. Ficolin-2 has been localized to chromosome 9 (9q34) and contains 288 amino acids producing a 35Kd protein after glycosylation. It is composed of a short N-terminal region implicated in multimer generation, and a collagen-like domain consist of a series of 19 (Gly-X-Y) repeats. Generally, the distribution of serum ficolin-2 is perfectly Gaussian in healthy adult individuals, thus the mean and median are the same. The value has been demonstrated to be between 3 and 4 µg/mL.
It is worth noting that ficolin-2 is one of the few molecules known to activate the lectin pathway of complement activation. The activation is arisen after producing a complex with MBL-associated serine proteases (MASP)-1, -2, and -3. MASP-2 is an important protein for complement activation and its binding takes place at a site on the collagen-like region. Ficolin-2-MASP-2 interaction results in activation of the latter, enabling it to cleave complement components C2 and C4 like the C1q, r, s complex of the classical pathway stated by antigen-antibody formation. L-ficolin has been reported to bind to the Salmonella typhimurium (Ra strain), Pseudomonas aeruginosa, Gram-negative bacteria, Gram-positive species, Escherichia coli, as well as the Staphylococcus aureus and streptococci. Besides, all interactions were partially sensitive to GlcNAc.
 Fig. 1 L-ficolin structure. Distributed under CC BY-SA 3.0, from Wiki, without modification.
Fig. 1 L-ficolin structure. Distributed under CC BY-SA 3.0, from Wiki, without modification.
Ficolin-2 Functional Service
Creative Biolabs delivers an extensive range of ficolin-2-centric products, including anti-ficolin-2 antibodies, ELISA kits, and human complement ficolin-2 proteins. These precisely engineered instruments are vital for advancing research efforts directed towards devising therapeutic strategies for a multitude of diseases.
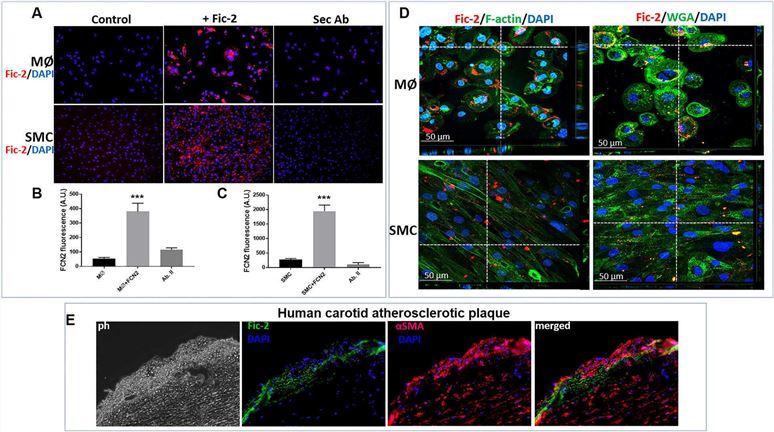 Fig.2 Immunofluorescence images illustrate the interaction between ficolin-2 and macrophages or smooth muscle cells.1
Fig.2 Immunofluorescence images illustrate the interaction between ficolin-2 and macrophages or smooth muscle cells.1
Ficolin-2 has been discovered in atherosclerotic plaques and linked to future cardiovascular incidents, though its precise role is still unclear. Researchers propose that it might affect plaque stability by modulating macrophage (MØ) and smooth muscle cell (SMC) interactions. To explore this hypothesis, an in-vitro co-culture system of SMC and MØ was treated with ficolin-2, followed by comprehensive analyses including cytokine and protease arrays, ELISA, qPCR, Western Blot, and monocyte migration assays. Findings revealed that ficolin-2 enhances pro-inflammatory responses, such as upregulating MCP-1, IL-6, and TLR4, activating key signaling pathways, and promoting monocyte transmigration. Immunofluorescence analysis of atherosclerotic plaques showed ficolin-2’s co-localization with SMC markers and cytokines, suggesting new pathways of inflammation associated with ficolin-2.
Creative Biolabs provides an extensive array of services related to ficolin-2, including binding assessments and further functional research, meticulously tailored to support their prestigious clients in both clinical and research endeavors.
References
-
Macarie, Răzvan Daniel, et al. "Ficolin-2 amplifies inflammation in macrophage-smooth muscle cell cross-talk and increases monocyte transmigration by mechanisms involving IL-1β and IL-6." Scientific Reports 13.1 (2023): 19431. Distributed under Open Access license CC BY 4.0, without modification.


 Datasheet
Datasheet Fig. 1 L-ficolin structure.
Fig. 1 L-ficolin structure. Fig.2 Immunofluorescence images illustrate the interaction between ficolin-2 and macrophages or smooth muscle cells.1
Fig.2 Immunofluorescence images illustrate the interaction between ficolin-2 and macrophages or smooth muscle cells.1
