Product List Background IL-1β Functional Service
Background
Interleukin-1 beta (IL-1β), a pro-inflammatory cytokine, is required for immune responses. It is produced primarily by activated macrophages, dendritic cells, and monocytes in response to pathogens or tissue damage. IL-1β acts through IL-1 receptors (IL-1R1 and IL-1R2), triggering intracellular signaling via NF-κB and MAPK pathways. This signaling regulates gene expression involved in inflammation, immune cell activation, and tissue repair. IL-1β is distributed widely throughout the body, influencing diverse tissues and organs. Dysregulation of IL-1β signaling contributes to inflammatory disorders such as rheumatoid arthritis and inflammatory bowel disease, making it a significant target for therapeutic intervention.
Its Gene ID: 3553, UniProtKB ID: P01584, and OMIM ID: 147720.
Molecular Mechanism of IL-1β Secretion and Activation
The secretion and activation of IL-1β involve a complex molecular mechanism. Initially, IL-1β is synthesized as an inactive precursor (pro-IL-1β) in response to inflammation-related stimuli. Activation typically occurs through the formation of a multiprotein complex called the inflammasome, which is composed of pattern recognition receptors (PRRs) such as NLRs, AIM2, RIG-I or pyrin, ASC (apoptosis-associated speck-like protein containing a CARD), and caspase-1. The PRR NLRP3 generates the best-characterized inflammasome. Within the inflammasome, caspase-1 cleaves pro-IL-1β into its mature and bioactive form, IL-1β. This mature cytokine is then released from the cell via a non-classical secretion pathway involving vesicular transport or membrane pores. IL-1β exerts potent pro-inflammatory effects, contributing to immune responses and inflammation in various disease contexts.
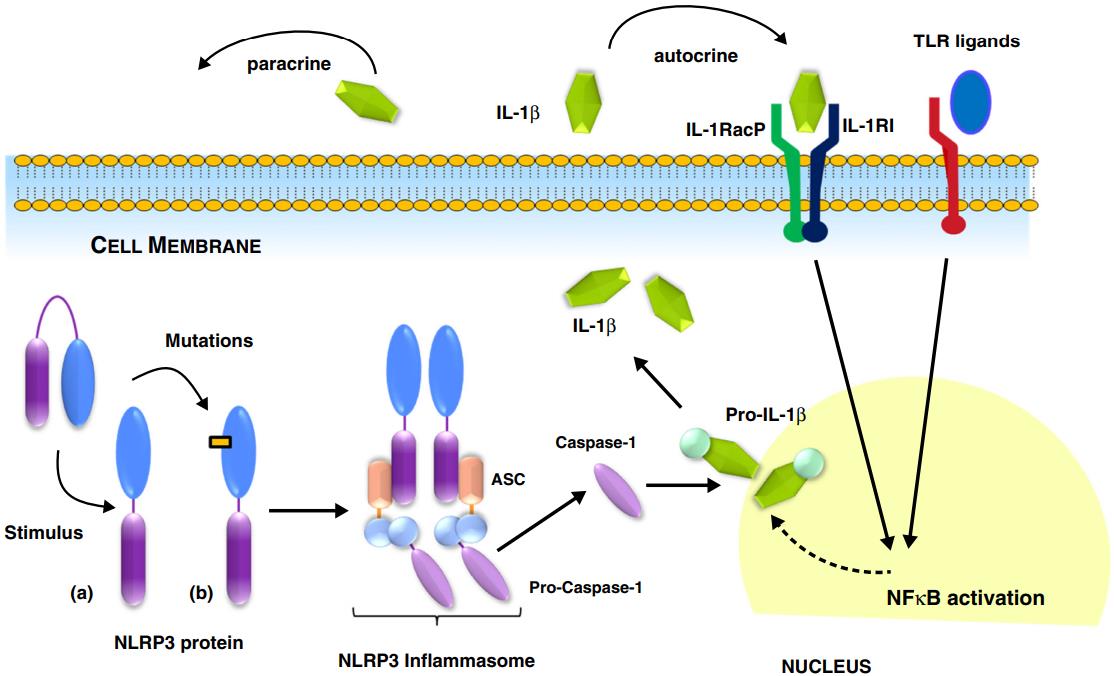 Fig.1 Production of mature IL-1β is regulated by the inflammasome.1, 4
Fig.1 Production of mature IL-1β is regulated by the inflammasome.1, 4
IL-1β and Diseases
IL-1β plays a critical role in various diseases: In autoinflammatory disorders, excess IL-1β leads to excessive inflammation, as seen in diseases like familial Mediterranean fever. In metabolic syndrome, IL-1β contributes to insulin resistance and vascular dysfunction. During acute inflammation, IL-1β can enhance various immune responses against infections. In chronic inflammation, sustained IL-1β activity contributes to tissue damage and the progression of diseases like rheumatoid arthritis. IL-1β also promotes tumor progression by supporting tumor cell proliferation, metastatic, angiogenesis, and immune evasion mechanisms.
Antibodies Targeting IL-1β
Antibodies targeting IL-1β are pivotal in both diagnostic and therapeutic applications. They can be employed in assays like ELISA to quantify IL-1β levels, aiding in disease diagnosis and monitoring studies. Besides, these antibodies can neutralize IL-1β, inhibiting its biological activity by blocking its interaction with IL-1 receptors, thereby attenuating downstream inflammatory signaling pathways. By modulating IL-1β activity, these antibodies help relieve symptoms and slow the progression of the diseases. Several IL-1β signaling antibody inhibitors have been intensively studied or clinically approved. A humanized IgG1 antibody targeting IL-1β has shown efficacy against several inflammatory diseases and is approved for treating cryopyrin-associated periodic syndromes.
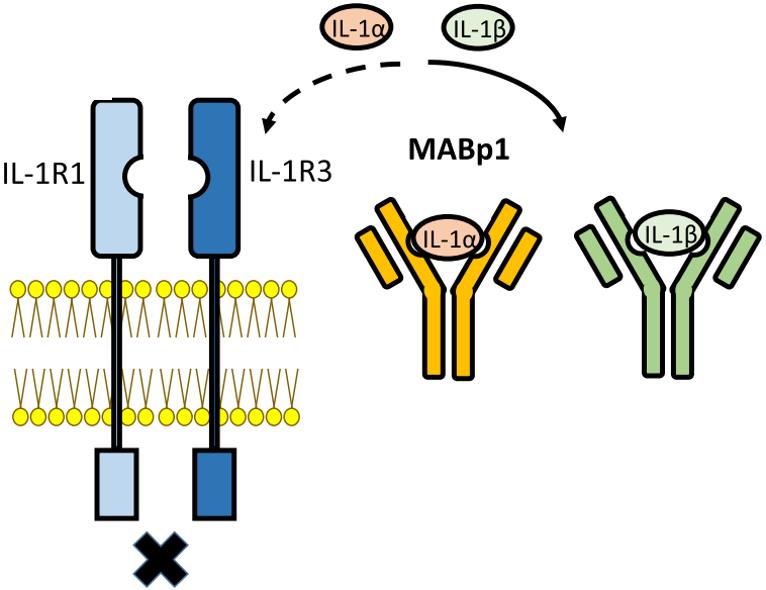 Fig.2 Illustration of the action of monoclonal antibodies against IL-1β.2, 4
Fig.2 Illustration of the action of monoclonal antibodies against IL-1β.2, 4
Creative Biolabs provides the human IL-1β ELISA kit and related products that have been validated for specificity, which helps to detect the target protein stably, reliably, and consistently.
IL-1β Functional Service
Creative Biolabs provides an array of reagents pertaining to IL-1β, featuring ELISA kits designed for detecting IL-1β. These instruments are proficient in uncovering and examining the interactions associated with the human IL-1β protein alongside multiple other entities. As a result, they represent indispensable tools in research focused on developing therapeutic approaches for disease intervention.
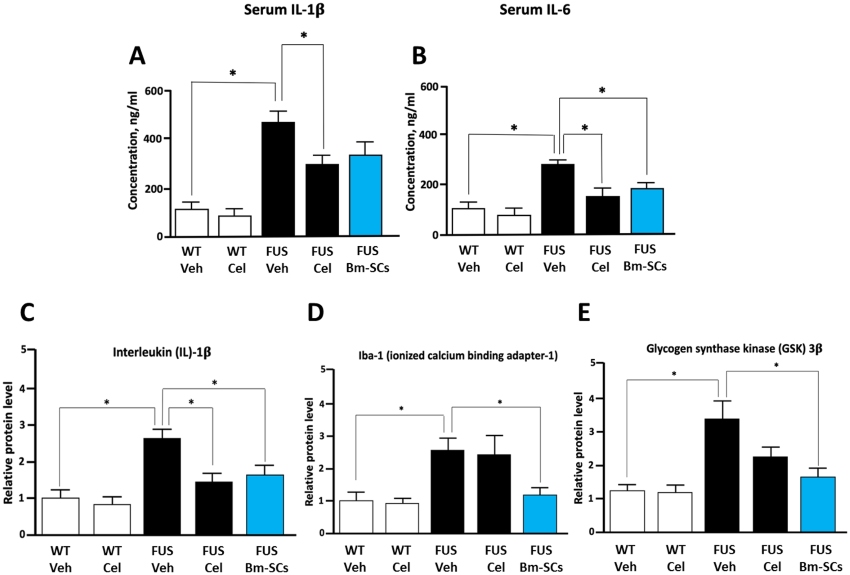 Fig.3 Analysis of ELISA-determined serum levels of IL-1β and IL-6, alongside Western blot assessment of spinal IL-1β, Iba-1, and GSK-3β in WT and FUS-transgenic mice post-treatment.3, 4
Fig.3 Analysis of ELISA-determined serum levels of IL-1β and IL-6, alongside Western blot assessment of spinal IL-1β, Iba-1, and GSK-3β in WT and FUS-transgenic mice post-treatment.3, 4
Neurodegenerative disorders often converge on inflammation-induced apoptosis or necrosis, regardless of their origins. Researchers suggested that bone marrow-derived stromal cells (bm-SC) interventions might be promising. Recent animal studies demonstrated that intrathecal transplantation of bm-SCs improved functional outcomes and decreased inflammation, unlike anti-inflammatory drugs. Notably, these benefits were observed in both rats with acute traumatic spinal cord injury and mice with chronic neurodegenerative conditions. The mechanism is thought to involve bm-SCs modulating glycogen synthase kinase-3β towards anti-apoptotic levels. This multipathway hypothesis is supported by reduced pro-inflammatory interleukin-1β, tumor necrosis factor, and activated microglia marker ionized calcium binding adapter-1 levels detecting by ELISA.
Creative Biolabs provides expert analysis in IL-1β functionality by conducting detailed studies of interaction dynamics and comprehensive functional evaluations. These tailored services meet the unique needs of esteemed clients, thereby greatly advancing clinical and scientific research efforts.
References
-
Gram, Hermann. "Preclinical characterization and clinical development of ILARIS®(canakinumab) for the treatment of autoinflammatory diseases." Current Opinion in Chemical Biology 32 (2016): 1-9.
-
Kaneko, Naoe, et al. "The role of interleukin-1 in general pathology." Inflammation and regeneration 39 (2019): 1-16.
-
De Munter, J. P. J. M., et al. "Why do anti-inflammatory signals of bone marrow-derived stromal cells improve neurodegenerative conditions where anti-inflammatory drugs fail?" Journal of Neural Transmission 127.5 (2020): 715-727.
-
Distributed under Open Access license CC BY 4.0, without modification.


 Datasheet
Datasheet Fig.1 Production of mature IL-1β is regulated by the inflammasome.1, 4
Fig.1 Production of mature IL-1β is regulated by the inflammasome.1, 4
 Fig.2 Illustration of the action of monoclonal antibodies against IL-1β.2, 4
Fig.2 Illustration of the action of monoclonal antibodies against IL-1β.2, 4
 Fig.3 Analysis of ELISA-determined serum levels of IL-1β and IL-6, alongside Western blot assessment of spinal IL-1β, Iba-1, and GSK-3β in WT and FUS-transgenic mice post-treatment.3, 4
Fig.3 Analysis of ELISA-determined serum levels of IL-1β and IL-6, alongside Western blot assessment of spinal IL-1β, Iba-1, and GSK-3β in WT and FUS-transgenic mice post-treatment.3, 4
