Stem Cell-derived Exosome Application
- Endothelial Progenitor Cell Source
Introduction Advantages Applications Services FAQs
Endothelial progenitor cell-derived exosomes (EPC-Exos): A Molecular Mediator of Vascular Remodeling
Endothelial progenitor cells (EPCs), broadly defined as precursors to mature ECs, exert their pro-angiogenic effects through two principal mechanisms: direct differentiation and paracrine signaling. While EPCs can migrate to sites of vascular injury and differentiate into functional ECs, much of their regenerative capacity is attributed to their secretome, particularly exosomes—nanoscale extracellular vesicles (30–150 nm in diameter) encapsulating bioactive molecules such as proteins, lipids, and nucleic acids.
Endothelial progenitor cell-derived exosomes (EPC-Exos) have garnered significant attention as key mediators of paracrine signaling. They carry a diverse repertoire of bioactive cargo, including miRNAs (e.g., miR-126, miR-21-5p, miR-486-5p), integrins, and growth factors, which orchestrate cellular responses in target cells. Notably, EPC-Exos have demonstrated comparable, and in some cases superior, therapeutic efficacy to EPCs themselves in preclinical models of cardiovascular, renal, and metabolic diseases. The inherent advantages of EPC-Exos—low immunogenicity, ease of storage and transport, and the absence of ethical concerns associated with stem cell therapies—further underscore their translational potential. As a frontrunner in exosome research and application development, Creative Biolabs has been actively exploring the therapeutic potential of EPC-Exos while providing end-to-end solutions for exosome characterization, engineering, and functional validation.
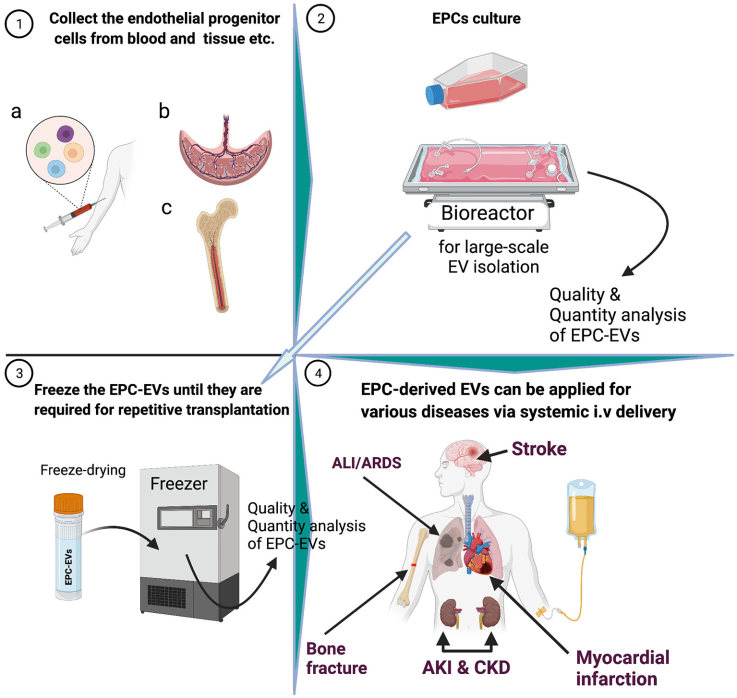 Fig.1 EPC-derived extracellular vesicles translation to the clinic.1
Fig.1 EPC-derived extracellular vesicles translation to the clinic.1
Unique Properties of EPC-Exos
EPC-Exos have emerged as a promising candidate for regenerative therapies due to their distinctive biological properties:
-
Angiogenesis Boost: These vesicles actively stimulate endothelial cell growth, migration, and blood vessel formation, making them particularly effective in treating ischemic injuries and degenerative diseases. Their ability to remodel damaged vascular networks surpasses conventional growth factor-based approaches.
-
Nano-Scale Delivery: With a compact size range of 30–150 nm, they penetrate tissues effortlessly and circulate systemically, offering a natural advantage for targeted drug delivery.
-
Built-In Stability: Encased in a protective lipid bilayer, EPC-Exos maintain structural integrity during storage and transportation, simplifying clinical logistics compared to fragile biologics.
-
Smart Targeting: Their surface molecules act like "biological GPS," enabling selective uptake by recipient cells through specific receptor interactions—a feature that enhances therapeutic precision.
-
Immune Stealth: The absence of MHC molecules allows them to evade immune detection, minimizing rejection risks in allogeneic applications.
-
Customizable Platform: Engineered to carry therapeutic payloads (e.g., drugs, miRNAs) or display tissue-specific homing signals, they serve as adaptable tools for personalized medicine. Notably, their derivation from progenitor cells avoids the ethical controversies surrounding embryonic or induced pluripotent stem cells.
Therapeutic Applications of EPC-Exos in Vascular Remodeling
EPC-Exos have shown remarkable potential in mitigating cardiac injury by promoting cardiomyocyte proliferation, enhancing neovascularization, and attenuating fibrosis. Molecular mediators such as integrin-linked kinase, Sonic Hedgehog, miR-363-3p, and miR-218-5p have been implicated in these effects. Studies have demonstrated improved cardiac function in models of myocardial infarction and heart failure following EPC-Exos administration. At Creative Biolabs, we offer advanced exosome profiling services, including exosomal RNA qPCR and miRNA sequencing, to elucidate the bioactive components driving these therapeutic outcomes.
Enriched with miR-126, EPC-Exos stimulate angiogenic signaling pathways (e.g., VEGF, AKT) in pulmonary microvascular ECs, promoting proliferation, migration, and angiogenesis. Beyond vascular repair, EPC-Exos modulate inflammatory responses, reduce pulmonary edema, and facilitate alveolar regeneration, making them promising candidates for conditions such as pulmonary fibrosis and chronic bronchitis. Creative Biolabs supports researchers with exosome engineering and targeting services to optimize EPC-Exos for respiratory disease models.
miR-126 abundant in EPC-Exos can target pulmonary microvascular EC (PMVECs) to activate downstream signaling pathways (such as VEGF pathway, AKT pathway, etc.) to promote the proliferation, migration, and angiogenesis of PMVECs. In addition, EPC-Exos can protect the damaged lung microstructure by reducing the alveolar inflammatory response and pulmonary edema and enhancing the regeneration ability of alveolar epithelial cells. Because of these effects, EPC-Exos has great potential in the treatment of pulmonary fibrosis, bronchitis, and other respiratory diseases. Creative Biolabs offers exosome profiling services such as qPCR analysis services and mass spectrometry-based analysis services to analyze the components of EPC-Exos.
EPC-Exos contribute to bone repair by promoting osteogenesis, enhancing angiogenesis within bone tissue, and modulating local inflammatory responses. Key components such as miR-126 and pro-angiogenic proteins facilitate vascularization and bone regeneration, offering a cell-free strategy for conditions like osteoporosis and fracture healing. Our exosome analysis services, including qPCR and mass spectrometry-based profiling, enable in-depth characterization of EPC-Exos components relevant to bone health.
EPC-Exos target renal tubular epithelial cells and vascular ECs, delivering miR-126, miR-486-5p, and anti-apoptotic factors that promote proliferation, reduce apoptosis, and suppress fibrosis. Their ability to restore vascular integrity and attenuate inflammation positions EPC-Exos as promising therapeutic agents in chronic kidney disease and acute kidney injury. Creative Biolabs offers proteomic profiling services to investigate the molecular mechanisms underlying these effects.
-
Diabetes and Complications
EPC-Exos modulate vascular health in diabetes by delivering miRNAs such as miR-126 and miR-296, which activate the PI3K pathway to support islet EC function and insulin secretion. Additionally, EPC-Exos accelerate wound healing in diabetic ulcers and improve microvascular complications by dampening inflammation and enhancing angiogenesis. Our exosome labeling services, using fluorescence or virus-based systems, enable in vivo tracking of EPC-Exos distribution and therapeutic outcomes in diabetic models.
One-Stop Services for EPC-Exos Research
At Creative Biolabs, we are dedicated to advancing the field of exosome therapeutics by offering comprehensive solutions tailored to EPC-Exos research:
Whether you aim to explore EPC-Exos for vascular regeneration or seek to engineer them as targeted delivery vehicles, Creative Biolabs is your trusted partner. Contact us today to discuss how we can support your research goals and help unlock the full therapeutic potential of EPC-Exos.
FAQs
Q: What exactly are EPC-derived exosomes (EPC-Exos)?
A: EPC-Exos are tiny extracellular vesicles released by endothelial progenitor cells (EPCs). Think of them as nature's delivery trucks—packed with bioactive cargo like proteins, RNAs, and lipids that help regulate vascular health and promote repair processes. They're a big part of how EPCs communicate with other cells, especially in damaged or ischemic tissues.
Q: How do EPC-Exos differ from using EPCs themselves in therapy?
A: While EPCs can differentiate into endothelial cells and form new blood vessels directly, EPC-Exos work more like messengers, delivering the signals needed to stimulate angiogenesis, reduce inflammation, and promote cell survival. The beauty of EPC-Exos is that they offer a cell-free option—safer, with lower immunogenicity, and easier to store and handle.
Q: Can EPC-Exos be engineered or modified?
A: Absolutely! EPC-Exos can be loaded with therapeutic molecules—like specific RNAs or drugs—or even surface-modified for targeted delivery. That's part of what makes them such a promising platform for regenerative medicine and precision therapy.
Reference
-
Salybekov, Amankeldi A., et al. "Latest advances in endothelial progenitor cell-derived extracellular vesicles translation to the clinic." Frontiers in Cardiovascular Medicine 8 (2021): 734562. Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only. Cannot be used by patients.
Related Services:

 Fig.1 EPC-derived extracellular vesicles translation to the clinic.1
Fig.1 EPC-derived extracellular vesicles translation to the clinic.1









