Stem Cell-derived Exosome Application
- MSC from iPSC Source
Introduction Advantages Applications Services FAQs
Introduction of Exosomes Secreted by iMSCs (iMSCs-Exos)
The advent of induced pluripotent stem cell (iPSC) technology has revolutionized regenerative medicine by offering a renewable, ethically non-contentious source of pluripotent cells capable of differentiating into various somatic cell types. Among these, mesenchymal stem cells derived from iPSCs (iMSCs) represent a significant innovation. These cells retain the hallmark characteristics of traditional MSCs—self-renewal and multipotency—while also offering a scalable and standardized alternative that circumvents many limitations associated with tissue-derived MSCs.
In recent years, exosomes secreted by iMSCs (iMSCs-Exos) have emerged as a promising cell-free therapeutic modality. These nano-sized vesicles, ranging from 30 to 150 nm in diameter, are loaded with diverse biomolecules such as RNAs, proteins, and lipids, reflecting the physiological state and functional capabilities of their parent cells. Importantly, iMSCs-Exos retain many of the therapeutic attributes of iMSCs while overcoming critical safety and regulatory challenges, positioning them as a viable candidate in translational medicine.
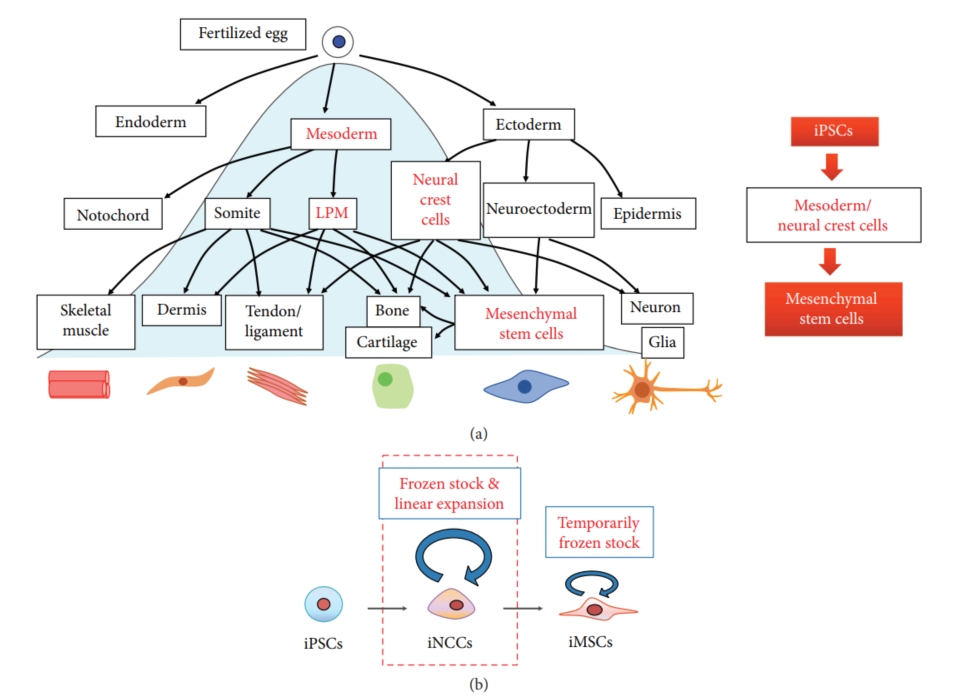 Fig.1 Derivation of MSCs from iPSCs.1
Fig.1 Derivation of MSCs from iPSCs.1
Creative Biolabs provides a comprehensive array of services for iMSC-derived Exosomes (iMSC-Exos), playing a pivotal role in advancing their therapeutic potential. These services include exosome isolation, purification, characterization, profiling, engineering, manufacturing, and functional testing in vitro and in vivo, specifically tailored for iMSCs-Exos, to ensure the highest quality and efficacy in research and development.
Limitations of iMSCs and the Emergence of Exosome-Based Therapies
While iMSCs hold considerable promise for clinical applications, several limitations hinder their therapeutic implementation:
-
Immunogenicity: Despite being derived from autologous cells, iMSCs can express foreign antigens or major histocompatibility complex (MHC) molecules, potentially triggering immune responses.
-
Genetic and epigenetic instability: Reprogramming and expansion procedures can introduce genomic aberrations and epigenetic drift, leading to clonal heterogeneity and increased tumorigenic risk.
-
Batch variability: Differences in culture conditions, reprogramming techniques, and differentiation protocols contribute to inconsistent cellular phenotypes and therapeutic outcomes.
-
Regulatory hurdles: The complexity of ensuring safety, potency, and efficacy through direct iMSC administration necessitates extensive preclinical evaluation.
In contrast, iMSC-derived exosomes (iMSC-Exos) present a promising alternative that circumvents many of the challenges associated with direct cell transplantation.
Importantly
-
Small size: Their small size—typically ranging from 30 to 150 nanometers—allows them to navigate biological barriers such as the blood-brain barrier with ease.
-
Minimal immunogenicity: iMSC-Exos exhibit inherently low immunogenicity, as they primarily function as mediators of intercellular communication rather than introducing foreign cells that might trigger immune rejection.
-
Ease of acquisition: as iMSCs can be expanded in vitro, exosomes derived from these cells can be produced in large, standardized quantities.
-
Abundant content: iMSCs-Exos are rich in a variety of bioactive cargos, including RNAs, proteins, and signaling molecules that contribute to diverse therapeutic effects—ranging from modulating inflammation and promoting angiogenesis to enhancing tissue repair and supporting cellular proliferation. At Creative Biolabs, we offer specialized services such as exosomal RNA isolation and qPCR analysis services and exosomal protein profiling services, which provide critical insights into the molecular mechanisms underpinning the therapeutic potential of iMSCs-Exos.
iMSC-Exos Applied to Therapy Due to Their Benefits
iMSCs-Exos have proved encouraging outcomes in preclinical investigations, indicating their latent force as a therapeutic choice for various diseases.
-
Visceral ischemia/reperfusion (I/R) injury
Preclinical models of hepatic and renal I/R injury have demonstrated the protective efficacy of iMSCs-Exos. These vesicles activate the sphingosine kinase/sphingosine-1-phosphate signaling axis, promoting cell survival, inhibiting apoptosis, and attenuating inflammatory responses. Such findings suggest their utility in acute organ injury settings, where rapid and targeted intervention is critical. Creative Biolabs supports these studies with advanced exosome profiling and circulatory system disease model construction service to elucidate molecular mechanisms and optimize therapeutic outcomes.
In murine models of hindlimb ischemia, iMSCs-Exos facilitate angiogenesis by enhancing endothelial cell proliferation and migration. These effects contribute to improved perfusion and tissue recovery, highlighting their potential in peripheral artery disease and tissue-engineering applications. Creative Biolabs offers tailored motor system disease model construction service and in vitro cell experiments, along with exosome engineering platforms, to enable precise investigation and therapeutic optimization.
-
Inflammation and Autoimmune Conditions
iMSCs-Exos exhibit potent anti-inflammatory properties. In osteoarthritis models, intra-articular injection of exosomes enhances chondrocyte function and cartilage repair. Additionally, in vitro studies reveal their capacity to suppress macrophage pyroptosis and inhibit pro-inflammatory cytokine release, suggesting utility in treating inflammatory lung conditions such as acute lung injury. Creative Biolabs provides exosome cytokine profiling services and exosome characterization via ELISA to evaluate and validate the anti-inflammatory potential of therapeutic exosomes.
iMSCs-Exos play a significant role in tissue regeneration, partly due to their enrichment in pro-regenerative molecules. In cutaneous wound healing models, these exosomes accelerate repair through enhanced fibroblast migration and collagen synthesis. Similarly, in orthopedic applications, they stimulate osteogenesis and neovascularization in bone defect and osteonecrosis models. Through Exosomal proteomics detection services and high-throughput RNA profiling, Creative Biolabs enables clients to dissect regenerative mechanisms and refine therapeutic strategies.
Complete Set of Stem Cell Exosome Research Services at Creative Biolabs
At Creative Biolabs, we recognize the transformative potential of iMSCs-Exos in biomedicine. Our one-stop exosome platform offers end-to-end services specifically optimized for iMSC-derived exosomes to support your research and development goals:
-
Exosome Isolation: Whether you're in the early discovery phase or preparing for preclinical validation, we offer scalable isolation solutions—from small-scale iMSCs-Exos extraction service to large-scale iMSCs-Exos extraction service.
-
Exosome Purification: We employ advanced purification techniques such as size exclusion chromatography (SEC), immunoaffinity, and ultracentrifugation methods to ensure high-purity exosome preparations suitable for therapeutic use.
-
Exosome Characterization: Comprehensive characterization services include western blotting, nanoparticle tracking analysis (NTA), and nanaflow to assess size distribution, surface marker expression, and protein content.
-
Exosome Profiling: Our exosomal RNA, protein, and cytokine profiling services provide deep insights into the therapeutic cargo of iMSCs-Exos, enabling precision therapeutic development and quality control.
-
Exosome Engineering: Enhance targeting and therapeutic efficacy through our exosome engineering services, including exosome surface labeling, targeted exosome modification, and exosome cargo loading with RNAs or small molecules.
If you're exploring the promise of iMSCs-Exos for therapeutic or diagnostic applications, please contact us. We'd love to collaborate. From proof-of-concept studies to clinical translation, Creative Biolabs is your trusted partner in advancing exosome science.
FAQs
Q: What are iMSCs-Exos and how do they differ from traditional MSC-derived exosomes?
A: iMSCs-Exos are extracellular vesicles derived from mesenchymal stem cells that have been differentiated from induced pluripotent stem cells (iPSCs). Unlike MSC-derived exosomes sourced directly from adult tissues, iMSCs-Exos offer a more consistent and scalable production pipeline, owing to the virtually unlimited self-renewal capacity and differentiation potential of iPSCs. This distinction is especially valuable for therapeutic applications that demand batch-to-batch consistency and large-scale manufacturing.
Q: Why are iMSCs-Exos considered safer than direct iMSC transplantation?
A: Direct administration of iMSCs carries risks such as immune rejection, tumorigenicity, and genetic instability. iMSCs-Exos, by contrast, retain the regenerative and immunomodulatory properties of iMSCs without introducing living cells into the body. Their small size, low immunogenicity, and bioactive cargo make them a safer alternative with fewer regulatory and biological hurdles.
Q: How does Creative Biolabs ensure the quality of iMSCs-Exos used in research?
A: Creative Biolabs employs a multi-step quality assurance pipeline that includes standardized protocols for exosome isolation, purification (e.g., SEC and immunoaffinity), and characterization (e.g., NTA, western blotting, and flow cytometry). We also provide detailed exosome profiling services—such as RNA, protein, and cytokine analyses—to verify the therapeutic content and consistency of each batch.
Q: Can exosomes be customized for targeted delivery?
A: Yes. Creative Biolabs offers comprehensive exosome engineering services, including targeted ligand display, surface modification, and therapeutic cargo loading. These strategies are designed to enhance the specificity, potency, and efficacy of exosome-based therapeutics for diverse indications.
Q: Do you offer disease-specific exosome evaluation models?
A: Absolutely. Creative Biolabs provides disease model construction services tailored to various organ systems, including circulatory, motor, and respiratory systems. These models facilitate the in vivo and in vitro validation of iMSCs-Exos, helping to decipher their mechanisms of action and accelerate the development of targeted therapies.
Reference
-
Zhao, Chengzhu, and Makoto Ikeya. "Generation and applications of induced pluripotent stem cell‐derived mesenchymal stem cells." Stem Cells International 2018.1 (2018): 9601623. Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only. Cannot be used by patients.
Related Services:

 Fig.1 Derivation of MSCs from iPSCs.1
Fig.1 Derivation of MSCs from iPSCs.1









