Colorectal Carcinoma-Targeting Adenovirus Construction Service
Colorectal carcinoma remains one of the most prevalent and challenging malignancies worldwide, with conventional therapies often limited by systemic toxicity and development of resistance. Adenovirus vectors have emerged as powerful vehicles for cancer gene therapy due to their high transduction efficiency, large transgene capacity, and natural ability to replicate in and lyse tumor cells. However, the broad tropism of conventional adenovirus vectors significantly limits their therapeutic efficacy and safety in colorectal carcinoma treatment. Our colorectal carcinoma-targeting adenovirus construction service represents a transformative approach that overcomes these limitations through sophisticated genetic engineering of viral tropism. By incorporating tumor-specific targeting peptides and tissue-specific regulatory elements, we create next-generation adenoviral vectors that specifically recognize and eliminate colorectal cancer cells while sparing normal tissues. This targeted approach enhances therapeutic efficacy, reduces off-target effects, and opens new possibilities for both monotherapy and combination treatment strategies in colorectal carcinoma management.
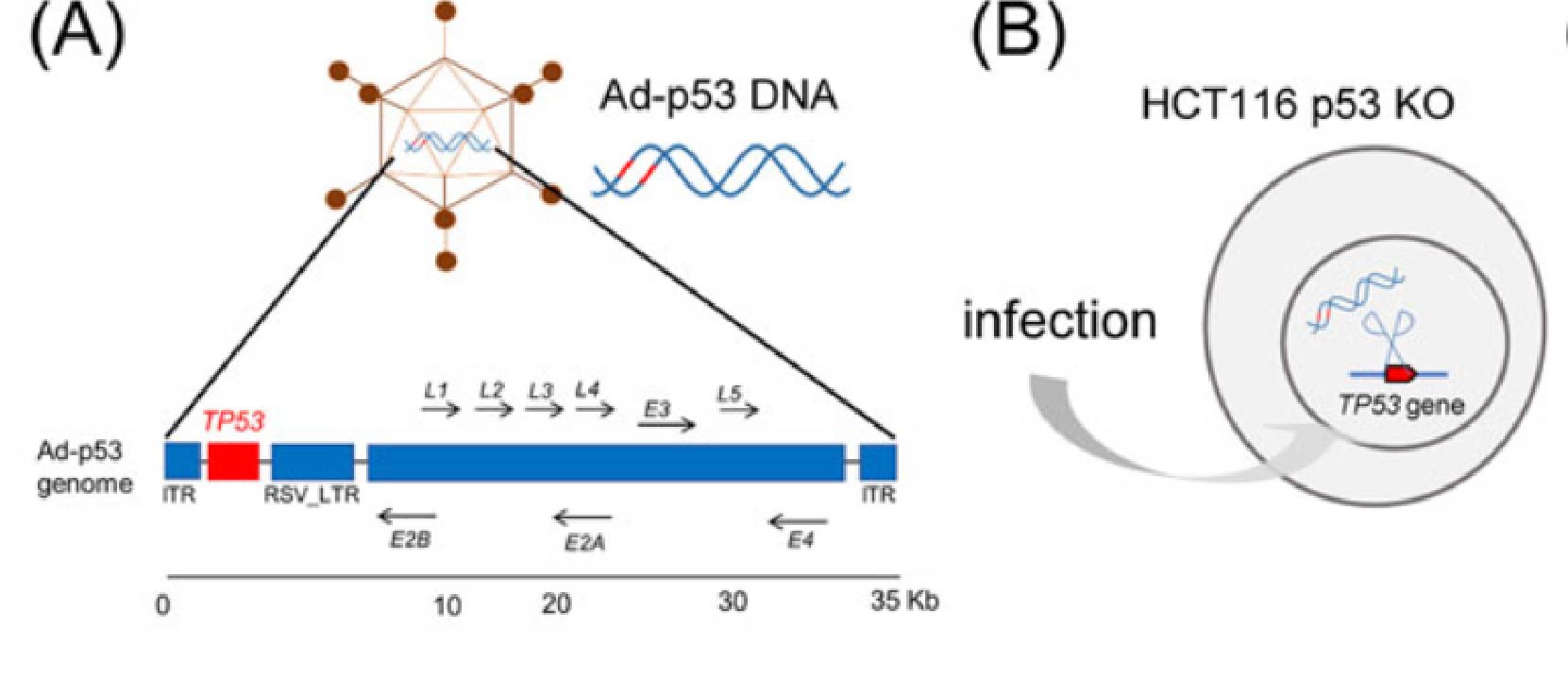 Fig.1 Schematic of the colorectal carcinoma-targeting p53 adenovirus vector; (B) Diagram of p53 adenovirus transfection in HCT116 p53 KO cells 1,2
Fig.1 Schematic of the colorectal carcinoma-targeting p53 adenovirus vector; (B) Diagram of p53 adenovirus transfection in HCT116 p53 KO cells 1,2
Our Comprehensive Service Workflow
We have established a systematic, phase-gated process to ensure the highest quality standards and successful project outcomes for our colorectal carcinoma-targeting adenovirus services. The process begins with an in-depth consultation to understand your specific therapeutic goals and experimental requirements. Our scientific team then proceeds with comprehensive bioinformatic analysis to identify optimal targeting strategies, including selection of tumor-specific peptides and tissue-restricted promoters. The design phase incorporates advanced molecular modeling to ensure proper folding and presentation of targeting motifs within the adenovirus capsid structure. Following vector construction using state-of-the-art molecular biology techniques, we proceed with iterative optimization and validation in relevant colorectal cancer models. The final products undergo extensive characterization to confirm targeting specificity, replication competence, and therapeutic efficacy before delivery to your laboratory.
Core Service Offerings
Targeting Strategy Design and Optimization
The success of a colorectal carcinoma-targeting adenovirus depends critically on the selection of appropriate targeting elements. Our service provides expert guidance in choosing the most effective targeting strategies for your specific application. We offer access to an extensive library of validated targeting peptides with demonstrated specificity for colorectal cancer biomarkers, including CEA, EpCAM, TAG-72, and guanylyl cyclase C. Additionally, we provide services for the identification and validation of novel targeting peptides through phage display screening against colorectal cancer cell lines and patient-derived tissues. Our optimization process includes comprehensive evaluation of peptide binding affinity, internalization efficiency, and functional activity in relevant biological systems. We also offer incorporation of protease-cleavable masking systems that further enhance tumor specificity through dual-targeting approaches.
Vector Engineering and Construction
Our molecular engineering capabilities form the foundation of our colorectal carcinoma-targeting adenovirus service. We employ multiple advanced strategies for creating tumor-selective vectors, including genetic incorporation of targeting peptides into adenovirus fiber knob domains, pIX proteins, and hexon hypervariable regions. For enhanced safety and specificity, we offer construction of transcriptionally targeted vectors utilizing colorectal cancer-specific promoters such as COX-2, CEA, and survivin promoters. Our platform also includes development of sophisticated conditionally replicative adenoviruses (CRAds) that incorporate multiple layers of tumor specificity through combinatorial targeting approaches. All constructed vectors undergo rigorous quality assessment, including full-genome sequencing, capsid protein expression analysis, and viral particle integrity verification.
Virus Production and Purification
We provide scalable adenovirus production services specifically optimized for colorectal carcinoma-targeting vectors. Our production platform utilizes advanced manufacturing processes in both adherent and suspension culture systems, with capacity ranging from small research batches to large-scale productions suitable for preclinical studies. The purification process employs multiple chromatography steps and ultracentrifugation techniques to ensure high purity and remove contaminants. Each production batch is accompanied by comprehensive documentation, including detailed records of production conditions and purification parameters. We also offer specialized services for the production of clinical-grade materials under appropriate quality systems for advanced therapeutic development.
Quality Control and Functional Validation
Quality assurance is integrated throughout our service pipeline, with particular emphasis on validating colorectal carcinoma targeting specificity. Our comprehensive quality control system includes physical characterization through electron microscopy and dynamic light scattering, chemical analysis using SDS-PAGE and mass spectrometry, and biological assessment through titer determination and sterility testing. Most importantly, we conduct extensive functional validation using relevant colorectal cancer models, including established cell lines, patient-derived organoids, and xenograft models. These validation studies assess targeting specificity, replication competence, oncolytic potency, and bystander effects to ensure the vectors meet the highest standards of performance and safety.
| Feature | Conventional Adenovirus | Colorectal Carcinoma-Targeting Adenovirus | Key Advantage |
|---|---|---|---|
| Targeting Specificity | Broad native tropism via CAR receptor | Precise targeting through engineered peptides and transcriptional control | Enables tumor-specific delivery while sparing normal intestinal epithelium |
| Therapeutic Index | Limited by hepatotoxicity and other off-target effects | Significantly enhanced through reduced non-specific transduction | Allows for higher effective doses with improved safety profile |
| Replication Control | Constitutive replication in permissive cells | Tumor-selective replication via tissue-specific promoters | Confines viral spread to malignant tissues, enhancing safety |
| Transgene Expression | Ubiquitous expression driven by viral promoters | Tumor-restricted expression via CRC-specific regulatory elements | Maximizes therapeutic transgene impact while minimizing systemic effects |
| Manufacturing Consistency | Standard production protocols | Optimized processes for targeted vector production | Ensures batch-to-batch reproducibility and maintained targeting function |
Comparative Advantages of Colorectal Carcinoma-Targeting Adenovirus
Therapeutic Applications and Mechanisms
Our colorectal carcinoma-targeting adenovirus vectors enable multiple innovative therapeutic approaches for colorectal cancer treatment. In oncolytic virotherapy applications, these vectors provide direct tumor cell lysis through selective replication and spread within malignant tissues. For gene therapy applications, they enable targeted delivery of therapeutic transgenes, including suicide genes, immunomodulatory factors, and tumor suppressor genes. The vectors also show great promise in combination therapy approaches, where they can be used to sensitize tumors to conventional chemotherapy and radiation therapy. Additionally, they serve as powerful platforms for cancer immunotherapy by enabling localized expression of immune-stimulatory molecules and tumor antigens. The targeted nature of these vectors also makes them ideal for advanced applications such as targeted delivery of CRISPR-Cas9 systems for precise genome editing in colorectal cancer cells.
Technical Innovations and Advantages
Our colorectal carcinoma-targeting adenovirus technology incorporates several key innovations that provide distinct advantages over conventional approaches. The integration of multiple targeting modalities creates vectors with enhanced specificity through complementary mechanisms. Our advanced capsid engineering ensures optimal presentation of targeting ligands while maintaining viral infectivity and stability. The modular design of our platform allows for rapid adaptation to evolving understanding of colorectal cancer biology and emerging therapeutic targets. Furthermore, our vectors maintain the inherent advantages of adenovirus platforms, including high transduction efficiency and large transgene capacity, while achieving unprecedented specificity for colorectal carcinoma cells. The technology also supports the development of sophisticated therapeutic strategies that respond to specific tumor microenvironment cues, further improving targeting precision in heterogeneous tumor masses.
Client Support and Collaborative Development
We believe that successful therapeutic development requires strong partnerships and continuous collaboration. Our scientific team provides comprehensive support throughout the project lifecycle, from initial design to final data interpretation. We assign a dedicated project manager with specific expertise in colorectal cancer biology to each client, ensuring effective communication and scientific alignment. Our experts are available for regular technical consultations, offering insights based on extensive experience with colorectal cancer models and targeted vector development. We also provide detailed technical documentation and regulatory support packages to facilitate advancement toward clinical applications. For clients requiring additional resources, we offer extended collaboration options, including assistance with preclinical study design, data analysis, and follow-up vector optimization.
Quality Systems and Regulatory Compliance
Our services are performed under rigorous quality management systems designed to meet the requirements of therapeutic product development. All procedures are documented in detailed standard operating procedures, and our personnel undergo regular training to maintain technical excellence. We implement comprehensive documentation practices ensuring complete traceability of materials and processes. Our facilities maintain state-of-the-art instrumentation with regular calibration and maintenance programs. For projects requiring regulatory compliance, we adapt our processes to meet specific guidelines and provide additional documentation to support regulatory submissions. Our quality team has extensive experience with FDA and EMA requirements for gene therapy products, providing valuable guidance for clients pursuing clinical development pathways.
Future Directions and Technology Enhancement
We are committed to continuous innovation in colorectal carcinoma-targeting adenovirus technology. Current development efforts focus on expanding our targeting toolkit to address colorectal cancer heterogeneity and resistance mechanisms. We are engineering novel vectors capable of targeting cancer stem cells and overcoming physical barriers in tumor microenvironments. Advanced transcriptional targeting systems responsive to hypoxic conditions and inflammatory signals are under development to enhance tumor specificity further. We are also creating smart vectors capable of regulated transgene expression and conditional replication in response to specific molecular cues. Our ongoing research collaborations with leading cancer centers ensure that we remain at the forefront of colorectal cancer biology and can incorporate the latest scientific insights into our vector design strategies.
What Our Clients Say
"The colorectal carcinoma-targeting adenovirus developed by Creative Biolabs demonstrated exceptional specificity in our preclinical models. The vector showed potent oncolytic activity in patient-derived xenografts while completely sparing normal intestinal organoids. The level of targeting precision achieved through their engineered capsid technology represents a significant advancement over conventional oncolytic viruses."
— David Chen, PhD, Senior Scientist
"As a biotech company developing combination therapies for advanced colorectal cancer, we needed a targeted delivery platform for our immunomodulatory transgenes. Creative Biolabs's vectors enabled tumor-restricted expression with minimal hepatic sequestration, resulting in enhanced anti-tumor immunity and complete responses in our syngeneic models. Their team provided outstanding scientific support throughout the optimization process."
— Jennifer Li, Director of Oncology Research
Why Choose Our Service?
Specialized Expertise in Colorectal Cancer Biology
Our team includes molecular oncologists and virologists with deep expertise in colorectal carcinoma pathogenesis and treatment resistance mechanisms. This specialized knowledge enables us to design targeted vectors that address the specific challenges of colorectal cancer therapy.
Comprehensive Therapeutic Development Support
We provide end-to-end solutions from initial vector design through preclinical validation, eliminating the need for multiple vendors and ensuring project continuity. Our integrated approach accelerates therapeutic development timelines and enhances program value.
Proven Success in Targeted Vector Development
With numerous successful projects completed for academic institutions, pharmaceutical companies, and biotech firms, we have demonstrated our ability to deliver effective targeting solutions for colorectal carcinoma applications.
Advanced Technological Platform
Our proprietary adenovirus engineering platform incorporates the latest advances in viral vector technology, synthetic biology, and cancer targeting strategies, ensuring that our clients benefit from cutting-edge scientific innovations.
Commitment to Quality and Compliance
Our rigorous quality systems and regulatory expertise provide assurance that developed vectors will meet the standards required for advanced therapeutic development and clinical translation.
Contact Us Today
To discuss how our colorectal carcinoma-targeting adenovirus construction service can advance your therapeutic development program, please contact our scientific team. We welcome the opportunity to learn about your specific requirements and develop customized solutions that address the unique challenges of colorectal cancer treatment. Our team is available to provide detailed technical consultations, project quotations, and comprehensive information about our service capabilities and technology platform.
References
- Ning, Duo, et al. "Chromatin structure and gene transcription of recombinant p53 adenovirus vector within host." Frontiers in Molecular Biosciences 12 - 2025 https://doi.org/10.3389/fmolb.2025.1562357
- Distributed under Open Access license CC BY 4.0, without modification.
