Custom Herpes Simplex Virus Vector Production Service
Introduction
Creative Biolabs' Custom Herpes Simplex Virus Vector Production Service accelerates gene therapy research with advanced genetic engineering and robust production. Tailored for neurological disorders and oncolytic applications, we deliver high-titer, high-purity HSV vectors with precise gene delivery. Overcoming vector design and production challenges, our service ensures efficient, reliable progress toward your research goals.
[Discover How We Can Help - Request a Consultation]
Custom Herpes Simplex Virus Vector Production Service
Introduction of Herpes Simplex Virus Vector
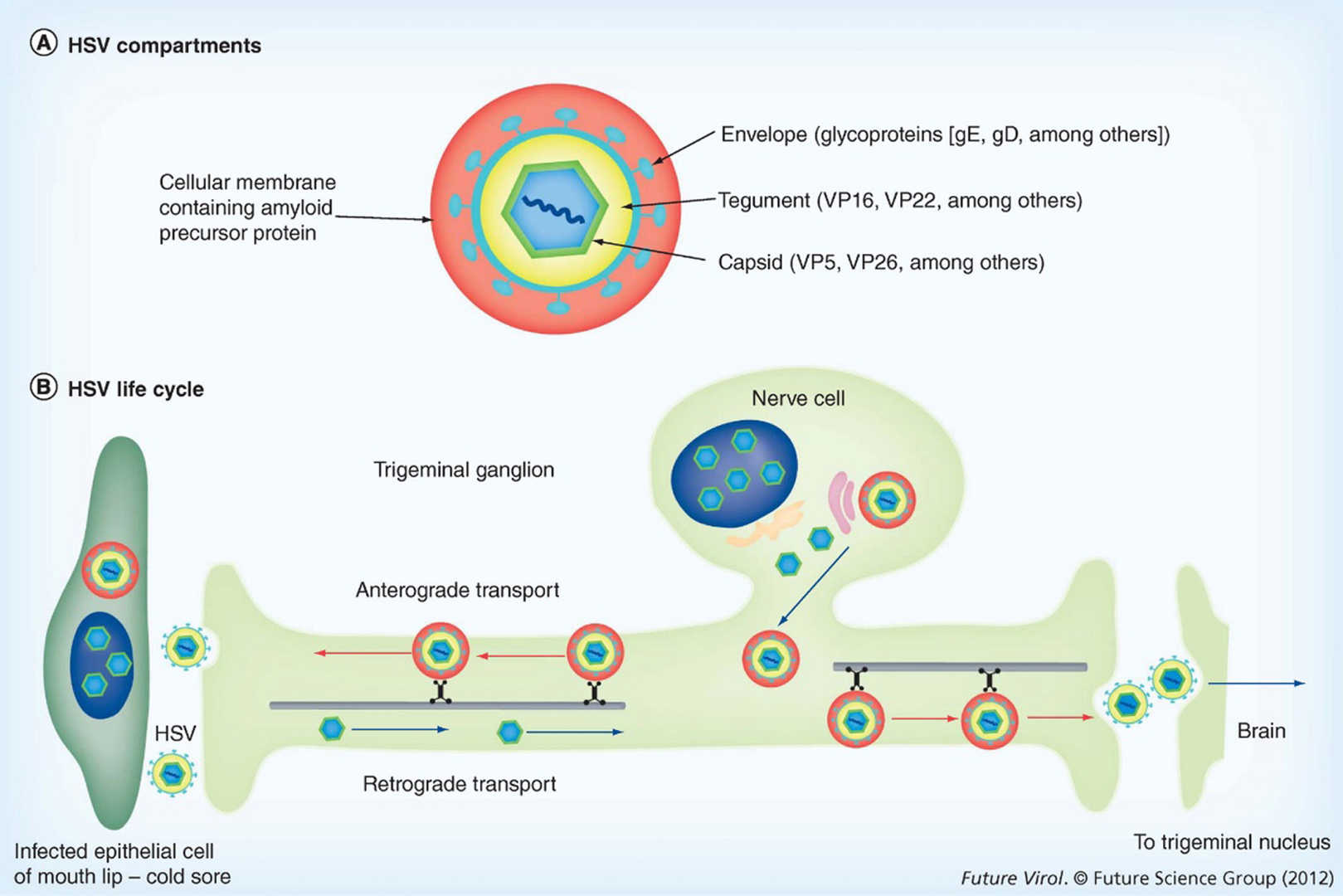 Fig.1 The components and life cycle of HSV.1,3
Fig.1 The components and life cycle of HSV.1,3
Herpes Simplex Virus type 1 (HSV-1) is a neurotropic, enveloped human pathogen widely utilized as a gene delivery vehicle. Its key characteristics make it highly suitable for gene therapy:
- Large Genome Capacity: HSV-1 has a large double-stranded DNA genome (around 152 kbp), allowing for the insertion of significant genetic material, including multiple transgenes or entire genomic loci (up to ~150 kbp for amplicons).
- Neurotropism: HSV-1 naturally infects and establishes latency in neurons, making it an ideal vector for neurological disorders. It can be efficiently transported retrogradely along axons to the neuron nuclei.
- Non-Integrative: HSV vectors typically remain as extrachromosomal episomes in the host cell nucleus, minimizing the risk of insertional mutagenesis.
- Low Immunogenicity: Engineered HSV vectors exhibit low immunogenicity, allowing for repeated administrations without significant immune rejection.
Two major types of non-replicative HSV-1 vectors are primarily used in gene therapy:
- Non-Replicative Genomic Vectors (nrHSV-1): These vectors carry a modified virus genome deleted in at least one essential immediate-early (IE) gene, preventing lytic replication in normal cells. Modern nrHSV-1 vectors are engineered to be deficient in the expression of all IE genes, significantly reducing cytotoxicity and inflammation, while maintaining robust and durable transgene expression.
- Amplicon Vectors: These are helper-dependent vectors whose genomes do not carry any viral lytic genes. Instead, they consist of a concatemeric DNA plasmid (amplicon plasmid or minicircle) containing the transgene, an origin of DNA replication, and a packaging signal. Amplicons offer an exceptionally large cargo capacity and minimal viral protein expression, leading to very low toxicity and immunogenicity.
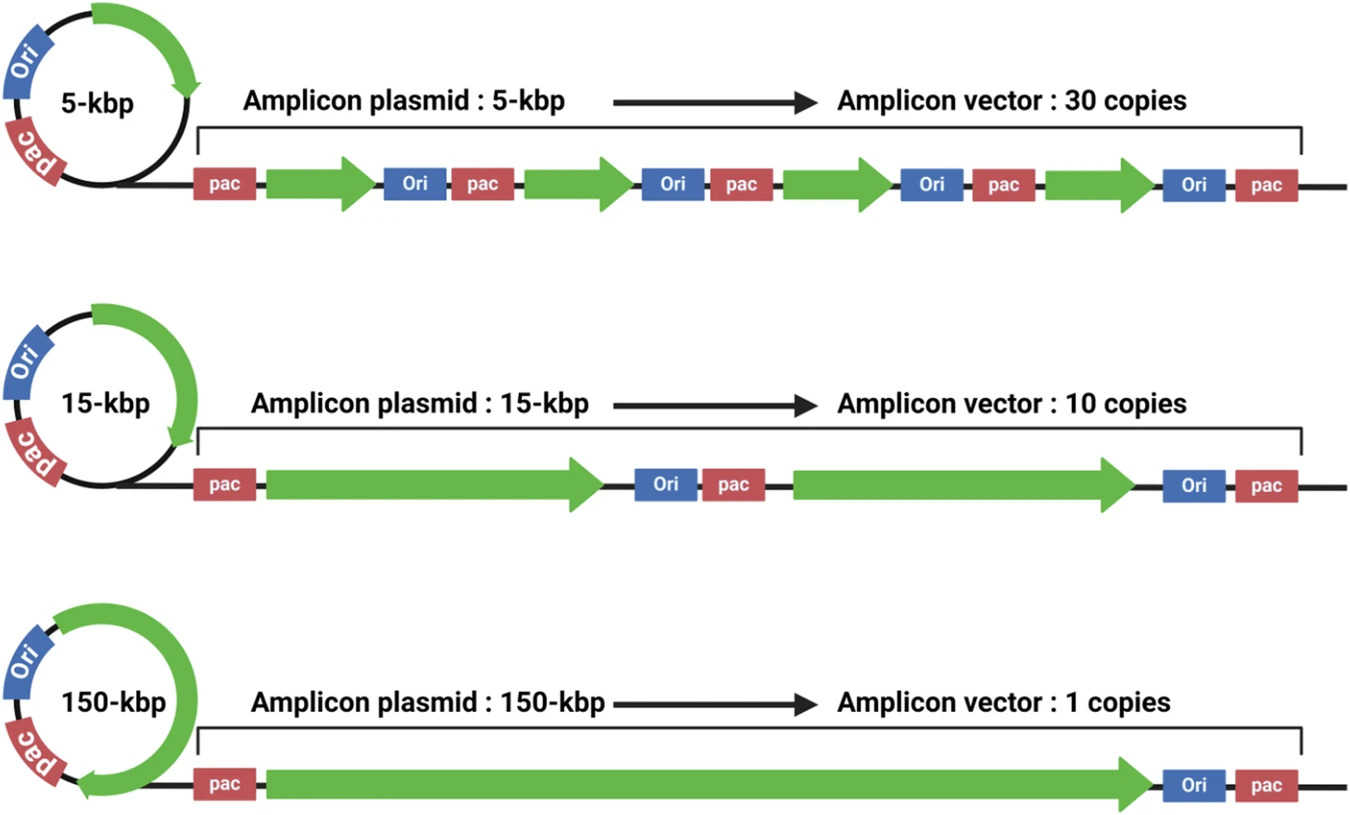 Fig.2 Amplicon vectors can deliver plasmids of different sizes as tandem repeats.2,3
Fig.2 Amplicon vectors can deliver plasmids of different sizes as tandem repeats.2,3
Workflow
-
Produce
- For nrHSV-1 Vectors: These are produced in complementing cell lines that express the essential viral functions missing from the vector. We utilize advanced cell lines to optimize vector titer.
-
For Amplicon Vectors: The main challenge has been helper virus contamination:
- BAC-based Co-transfection: Co-transfect amplicon plasmids with BAC carrying a defective HSV-1 helper genome to prevent helper genome packaging, ensuring high-purity vectors.
- Cre/loxP-based Recombination: Conditional deletion of helper virus packaging signals in Cre-expressing cells reduces contamination to 0.5-1.0%. Employs minicircle amplicons (bacterial DNA-free) to avoid transgene silencing and enhance expression.
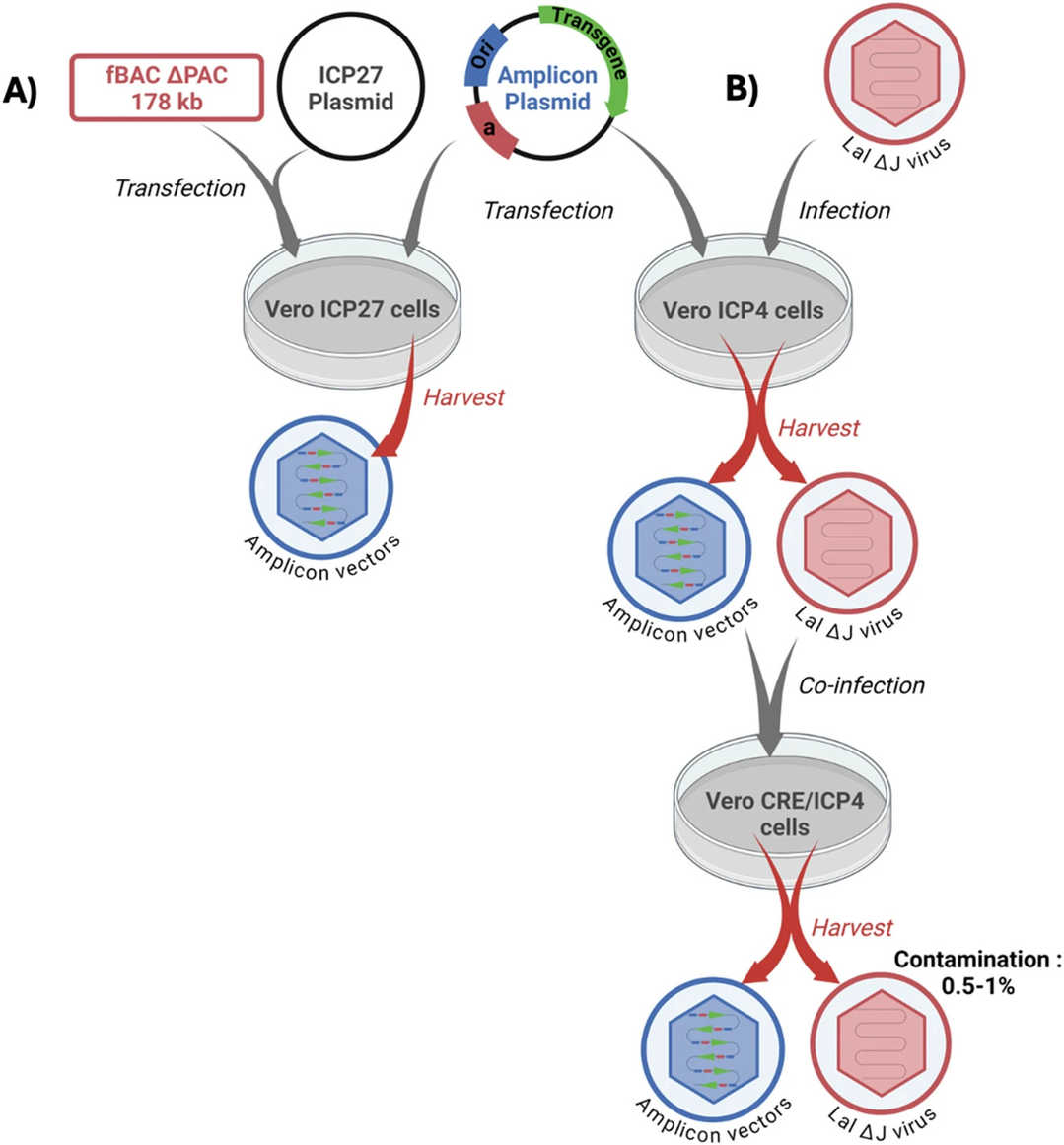 Fig.3 Different systems for the production of amplicon vectors.2,3
Fig.3 Different systems for the production of amplicon vectors.2,3
-
Purification
- Sucrose Density Gradient Centrifugation: Purifies and concentrates HSV vectors by separating them from cellular debris, residual helper virus, and impurities, ensuring ultra-pure preparations for sensitive in vitro/in vivo applications.
-
Quality Control
- Titer Determination: Accurate quantification of infectious viral particles (e.g., plaque-forming units, pfu/mL).
- Purity Assessment: Evaluation of helper virus contamination levels using sensitive molecular methods.
- Sterility Testing: Confirmation that the vector preparation is free from bacterial, fungal, and mycoplasma contamination.
- Identity Verification: Molecular techniques to confirm the presence and integrity of the transgene within the vector.
- Functional Validation: Optional assays to confirm transgene expression and biological activity in relevant cell lines or in vivo models, ensuring the vector performs as expected.
What We Can Offer
Our Custom Herpes Simplex Virus Vector Production Service at Creative Biolabs is designed to empower your research with unparalleled flexibility and precision.
[Experience the Creative Biolabs Advantage - Get a Quote Today]
Customer Reviews

FAQs
Q1: How to address the genomic instability of HSV vectors during continuous passage?
A: Due to the high GC content (≈68%) and repeat sequences of the HSV genome, we employ:
- BAC cloning technology: Clone the HSV genome into BACs for precise editing via RecA-mediated homologous recombination, reducing random recombination.
- Temperature-sensitive mutation: Introduce the tsK mutation in the UL54 (ICP27) gene to suppress lytic replication at 39°C, allowing only latent state maintenance.
- Sequencing monitoring: Perform PacBio long-read sequencing every 5 passages to detect genomic rearrangement hotspots (e.g., a sequences and LAT regions).
Q2: How to balance oncolytic activity and immunogenicity of HSV vectors in immunotherapy?
A: Our precision regulation strategies include:
- Oncolytic gene expression control: Drive ICP34.5 gene with an MHC-I promoter for high expression in tumor cells (promoting lysis) and silencing in normal cells.
- Bifunctional immune checkpoint design: Display PD-L1 antibody fragments on the vector surface and express IL-12 in the viral genome to enhance anti-tumor immunity.
- Dose optimization: Low MOI (0.1) infection induces immunogenic cell death, while high MOI (10) directly lyses tumors, adjusted by tumor type.
Q3: How to leverage HSV vector replication to reprogram the tumor microenvironment metabolism?
A: We developed a metabolic-regulating oncolytic HSV system:
- Glutaminase gene insertion: Insert the human glutaminase 2 (GLS2) gene into the HSV genome, driven by a tumor-specific hTERT promoter to deplete glutamine in the tumor microenvironment and inhibit cancer cell proliferation.
- Lactate dehydrogenase knockout: Delete the UL13 gene of HSV-1 to induce lactate accumulation in tumor cells, acidifying the microenvironment to enhance immune cell infiltration.
- Combination therapy: This vector combined with PD-1 antibody achieves 65% tumor regression in a colorectal cancer mouse model, 3-fold higher than monotherapy.
Q4: How to remove residual bacterial DNA contamination during HSV vector production?
A: Our three-step purification process includes:
- Benzonase treatment: Add 25 U/mL Benzonase to cell lysates, incubate at 37°C for 2 hours to degrade linear double-stranded DNA.
- Density gradient centrifugation: Use OptiPrep gradient (15%-40%) for ultracentrifugation (100,000×g) to separate virus from bacterial DNA.
- Affinity chromatography: Bind HSV to heparin-Sepharose column, allowing bacterial DNA to pass through, yielding 99.8% pure virus after elution (qPCR verified).
Q5: How to resolve local inflammatory responses induced by intramuscular HSV vector injection?
- Hydrogel encapsulation: Encase HSV vectors in PEG-PCL thermosensitive hydrogel, forming a sustained-release depot at 37°C.
- Local immunosuppression: Incorporate dexamethasone prodrug into hydrogel for local release within 24 hours post-infection, sparing systemic immunity.
- Serotype modification: Replace HSV-1 gC protein with HSV-2 homolog to reduce complement C3 deposition, decreasing inflammatory cell infiltration by 50%.
[Contact Our Team for More Information and to Discuss Your Project]
References
- Laurens, Weynants, et al. "Unlocking potential: Herpes Simplex Virus Type 1-based gene therapy in functional urology." Continence 10 (2024). DOI: 10.1016/j.cont.2024.101311
- Le Hars, Matthieu, et al. "Non-replicative herpes simplex virus genomic and amplicon vectors for gene therapy-an update." Gene Therapy (2024): 1-11. DOI: 10.1038/s41434-024-00500-x.
- Distributed under Open Access license CC BY 4.0, without modification.
