Immunoregulatory Molecules-loaded Oncolytic Vaccinia Virus
The size and the cloning flexibility of the oncolytic vaccinia virus (VACV) make it an attractive vector for delivering multiple genes to activate optimally antitumor immune responses. Creative Biolabs is one of the world's most trusted experts to provide oncolytic VACV development services.
VACV Encoding Immunoregulatory Molecules for Enhanced Antitumor Immunity
The important roles of cytokines and costimulatory molecules in promoting antitumor immune responses have been well documented. Cytokines play pivotal roles against multiple types of cancer; however, the clinical applications may be limited because of the severe toxicities associated with high doses delivered systemically. One approach to achieve the goal of continuous production of cytokines at the tumor site would be to deliver these cytokines by replication-competent vaccinia viruses.
Expression of immunostimulatory molecules may be another way to enhance the overall antitumor efficacy of the oncolytic virus. Oncolytic viruses have been extensively employed to deliver immune-activating cytokines, such as IL-2, IL-12, granulocyte-macrophage colony-stimulating factor (GM-CSF), TNF-α, and costimulatory molecules (such as B7.1, ICAM-1, and LFA-3), to optimally modify the immune microenvironment and improve the immune effects of the virus. As a leading service provider in the oncolytic VACV field, Creative Biolabs provides one-stop solutions for developing immunoregulatory molecules-loaded oncolytic VACV. Taking advantage of the state-of-the-art platforms, our experienced staff can assist every customer in solving your problems.
- IL-2
To develop more effective immunotherapy using IL-2, researchers engineered several constructs expressed by a tumor-selective, oncolytic VACV. In addition, researchers have evaluated the efficiency of recombinant VACV expressing interleukin-2 (VV-IL-2) as a tumor vaccine in an immunocompetent mouse model of cancers. Treatment with this recombinant virus resulted in a reduced number of pulmonary metastases, improved survival, and minimal toxicity in a murine tumor model. These findings suggest that using the VACV to insert other heterologous gene combinations may provide a powerful and less toxic approach for novel vaccination strategies in treating and preventing cancer.
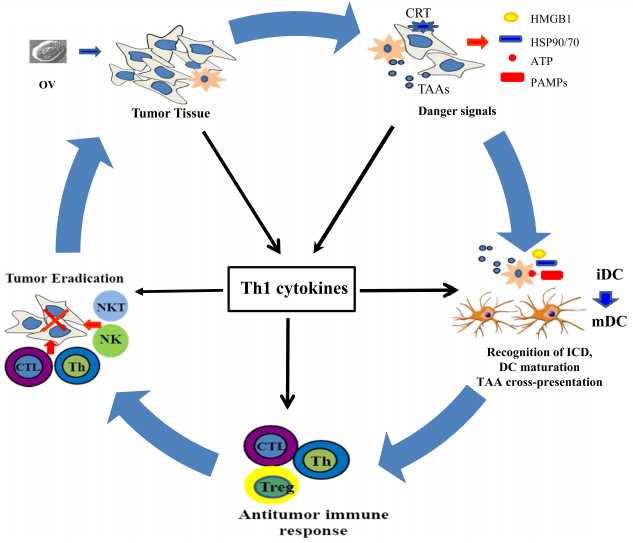 Fig.1 Immune-stimulatory molecules expressed by recombinant OV to potent antitumor immunity. (Guo, 2019)
Fig.1 Immune-stimulatory molecules expressed by recombinant OV to potent antitumor immunity. (Guo, 2019)
- IL-12
IL-12 is another cytokine that induces a potent antitumor effect by promoting natural killer (NK) cell and cytotoxic T cell activities. However, the expectations have been greatly diminished because systemic IL-12 in clinical trials evoked severe side effects such as leukopenia and thrombocytopenia. Oncolytic VACV delivering tethered IL-12 could turn a "cold" tumor into a "hot" tumor while avoiding systemic toxicity. The data demonstrated that tethered IL-12 could be maintained in the tumor without treatment-induced toxic side effects. Also, some researchers constructed a novel recombinant VACV that expresses IL-2 and both subunits of IL-12 along with a model tumor antigen. These vaccine constructs provided local cytokine release, effective anti-tumor therapy, and minimal toxicity in treating a model murine tumor. The use of recombinant VACV to express multiple cytokines that enhance anti-tumor responses represents a powerful technique of generating local cytokine expression with adjuvant properties for anti-tumor vaccines.
- GM-CSF
GM-CSF is an immunostimulatory cytokine that enhances immune-stimulatory effects. The oncolytic VACV, armed with an immunomodulatory gene encoding GM-CSF, has been tested in phase I clinical trials to stimulate the immunotherapeutic effects. Multiple intratumoral injections of VACV-GM-CSF were safe and the function of GM-CSF was maintained, and effective tumor regression was induced. The previous study has shown that the VACV-GM-CSF virus significantly enhanced oncolysis in the anon-Hodgkin lymphoma xenograft model compared to the parent virus. More recently, completed phase I and II clinical trials on patients with solid tumors have demonstrated that VACV-GM-CSF can be successfully delivered intravenously or intratumorally. The virus was well tolerated, and dose-dependent therapeutic effects were observed; these were correlated with increased survival. Thus, VACV-GM-CSF achieves its efficacy through direct oncolysis, or by induced antitumor immunity augmented by GM-CSF, and through the anti-vascular effects of the virus in tumor beds.
Creative Biolabs has long-term devoted to the development and application of the oncolytic VACV. Our scientists are confident in offering the most suitable solution for our customers worldwide.
Reference
- Guo, Z. S.; et al. Vaccinia virus-mediated cancer immunotherapy: cancer vaccines and oncolytics. Journal for immunotherapy of cancer. 2019, 7(1), 1-21.
