Why is the C1q Binding Assay So Important?
C1q, the first component of the classical complement pathway, plays a pivotal role in immune surveillance by binding to immune complexes and triggering downstream cascade activation. Understanding whether your biologic–be it a monoclonal antibody, Fc-fusion protein, or antigen–antibody complex–binds to C1q is crucial in multiple research contexts, including therapeutic antibody development, immunogenicity assessment, vaccine and adjuvant research, and autoimmunity & inflammation studies. A robust C1q binding assay provides functional insights well beyond traditional affinity or structural testing.
Great Minds Choose Creative Biolabs
Customization Options
Every project is different—so is every assay we run.

Species Customization
Human, mouse, rat, rabbit, and NHP C1q protein options are available.

Ligand Orientation Flexibility
Ligands can be immobilized (direct or indirect) or used in solution-phase format.

Source Control
Use of native or recombinant C1q, client-supplied reagents is supported.

Bundle Testing
Combine with CDC, ADCC, or FcγR binding for complete Fc functionality analysis.
Start Your Project
Workflow of Complement C1q-Binding Assays
We have briefly summarized the workflow for testing several complement components. For more information or customized service solutions, please feel free to contact us.
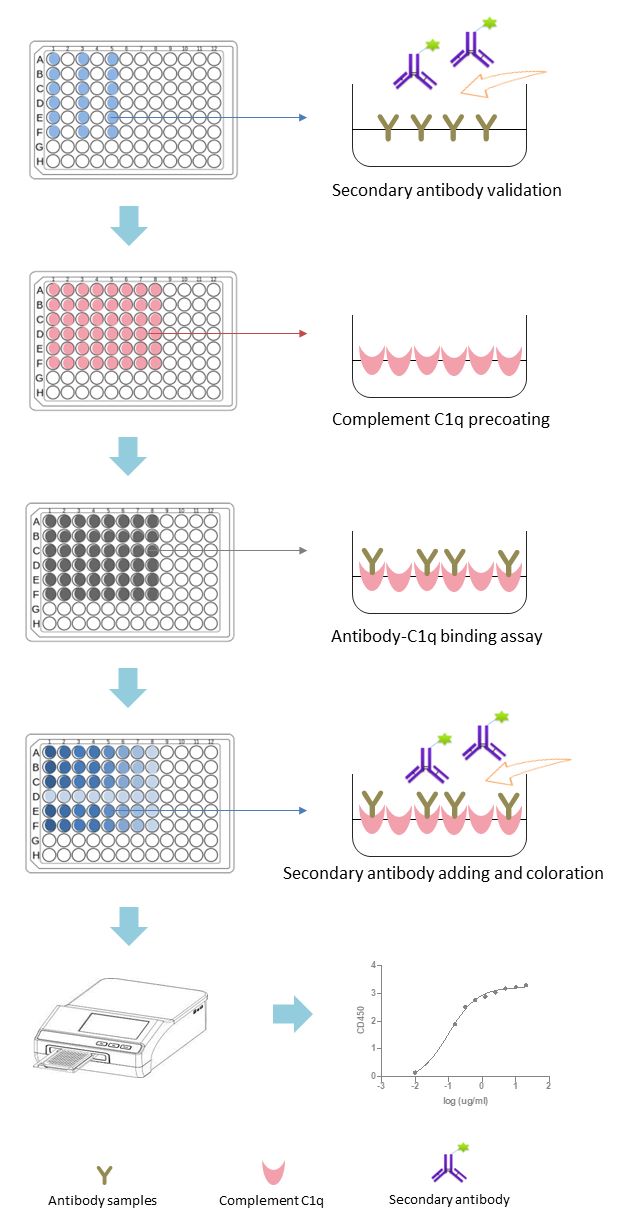 Fig. 1 Workflow of complement C1q-binding assays. (Creative Biolabs)
Fig. 1 Workflow of complement C1q-binding assays. (Creative Biolabs)
Design Your Workflow
Case Studies of C1q-Binding Assays
Case 1
C1q ELISA assay
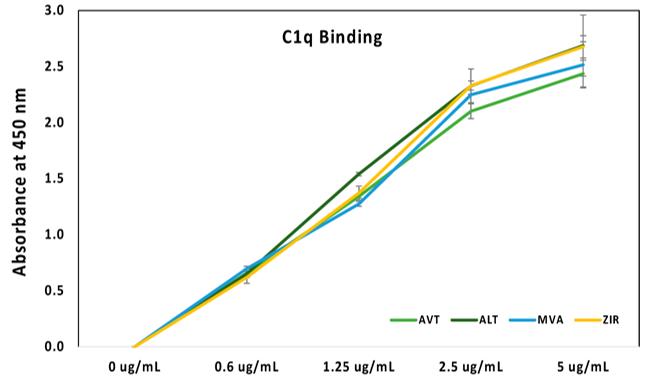
In one study, the C1q binding efficiency of four samples was analyzed using direct ELISA under the same conditions. The test found that the C1q binding rate increased with increasing antibody concentration. Four different C1q concentrations (0, 0.6, 1.25, 2.5, 5 ug/mL) were used, and the C1q binding showed linear increasing. On the other hand, it was found that the binding efficiencies of the four samples were similar, with no significant differences between them.
Fig. 2 C1q binding analysis of the samples.
1,3
Case 2
SPR analysis of the interaction between immobilized C1q and samples
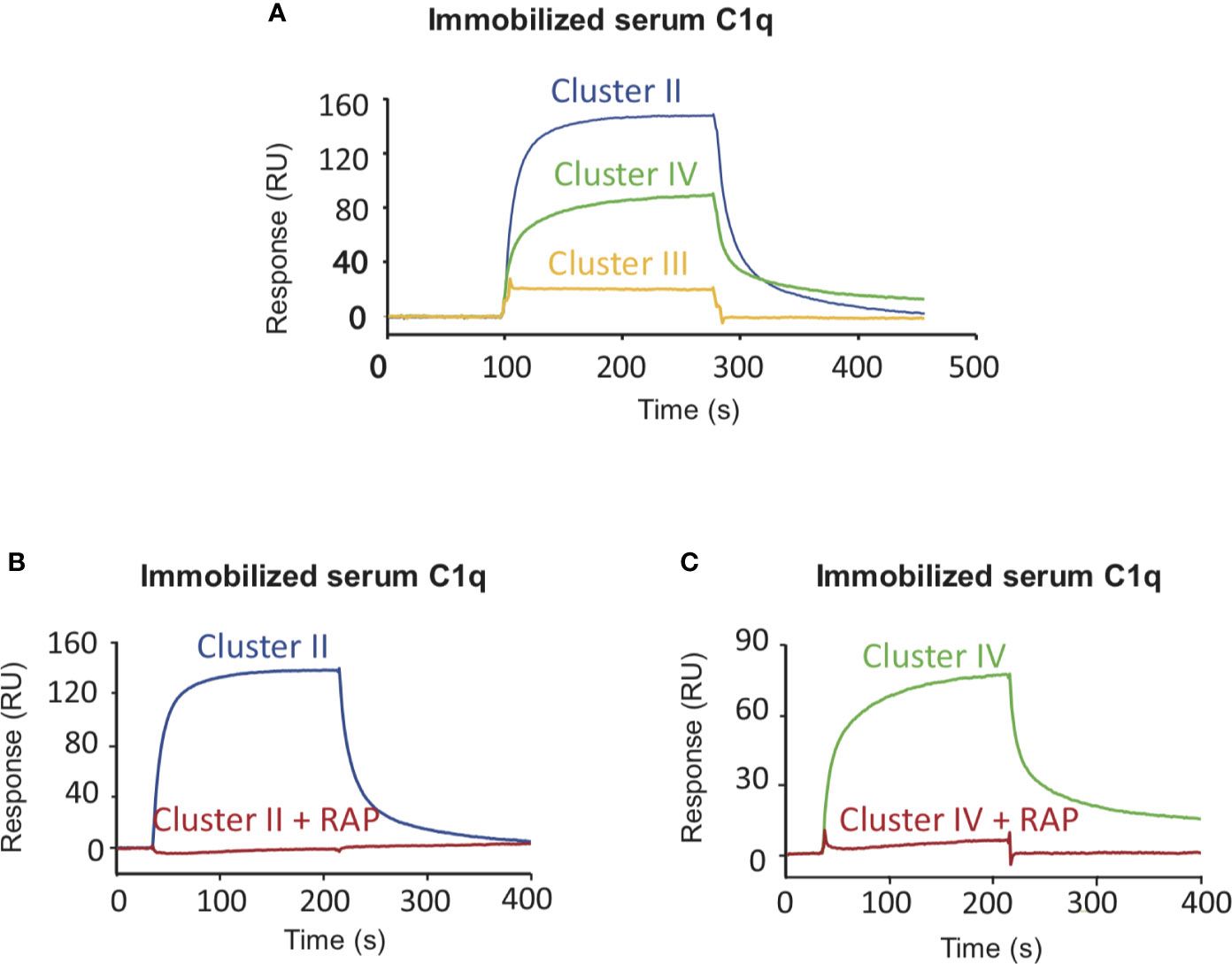
SPR analysis was performed on immobilized serum C1q injected with the sample. SPR kinetic analysis indicated the affinity of serum C1q for the sample.
Fig. 3 SPR analysis of samples interact with C1q.
2,3
References
-
Gürel, Büşra, et al. "Optimized methods for analytical and functional comparison of biosimilar mAb drugs: A case study for Avastin, Mvasi, and Zirabev." Scientia Pharmaceutica 90.2 (2022): 36. https://doi.org/10.3390/scipharm90020036
-
Fouët, Guillaume, et al. "Complement C1q interacts with LRP1 clusters II and IV through a site close but different from the binding site of its C1r and C1s-associated proteases." Frontiers in Immunology 11 (2020): 583754. https://doi.org/10.3389/fimmu.2020.583754
-
Distributed under Open Access license CC BY 4.0, without modification.
FAQ about C1q-Binding Assays
What platform is your C1q binding assay based on? Is it customizable?
Our C1q-binding assay primarily uses the ELISA format. Customers can customize the antigen coating method, C1q source (human/animal), binding time, and detection channel based on the type of sample being studied (e.g., serum, purified antibodies, candidate drugs) to achieve maximum experimental sensitivity and applicability.
We have requirements for trace samples. Is your company's service suitable?
We are well aware of the limitations on sample size in early-stage projects, so we have optimized the C1q binding test process to enable analysis under extremely low starting conditions, ensuring highly sensitive detection results. At the same time, we welcome customers to communicate with us in advance regarding sample types and processing requirements so that we can provide you with a more suitable sample pretreatment plan.
What is the difference between C1q binding analysis and the complement-dependent cytolysis (CDC) assay?
C1q binding analysis primarily detects whether antibodies can bind to the C1q protein, which is the initial step of the classical complement pathway; the CDC assay further observes whether this binding induces cell lysis. In summary, C1q binding is a prerequisite for CDC effects but cannot independently prove that an antibody possesses cytolytic activity. The two are typically used in combination to comprehensively assess an antibody's complement-mediated function.
Can the C1q binding assay be combined with other complement assays?
Of course. We offer a one-stop complement function analysis platform that supports the combined design of multiple tests, including the C1q-Binding Assay, CDC Assay, C3/C5 cleavage fragment detection, C5b-9 membrane attack complex (MAC) deposition detection, and CH50/AP50 activity analysis, to help customers comprehensively assess the complement-mediated activity of antibodies and their potential safety risks.















 Fig. 1 Workflow of complement C1q-binding assays. (Creative Biolabs)
Fig. 1 Workflow of complement C1q-binding assays. (Creative Biolabs)