Introduction What We Can Offer? Why Choose Us? Published Data FAQs Related Products Services
Accelerate Your Research and Development!
Are you currently facing challenges with uncontrolled complement activation, difficulties in developing precise therapeutics, or the need for advanced C1s targeting strategies? Creative Biolabs' Complement Therapeutic Target-C1s solutions help you precisely modulate complement activity, accelerate therapeutic development, and achieve superior outcomes through cutting-edge antibody engineering, high-throughput screening, and robust preclinical validation.
Contact our team to get an inquiry now!
Introduction
The complement system, a crucial component of innate immunity, plays a vital role in host defense against pathogens and in the clearance of immune complexes and apoptotic cells. However, its uncontrolled activation can lead to severe tissue damage and contribute to the pathogenesis of numerous autoimmune, inflammatory, and rare diseases. Central to the initiation of the classical complement pathway is the C1 complex, which consists of one C1q molecule, two C1r molecules, and two C1s molecules. C1s is a serine protease that, upon activation of the C1 complex, cleaves C4 and C2, leading to the formation of the classical pathway C3 convertase (C4b2a). This convertase then cleaves C3, initiating a cascade of events that culminate in the formation of the membrane attack complex (MAC) and subsequent cell lysis, as well as the generation of potent inflammatory mediators.
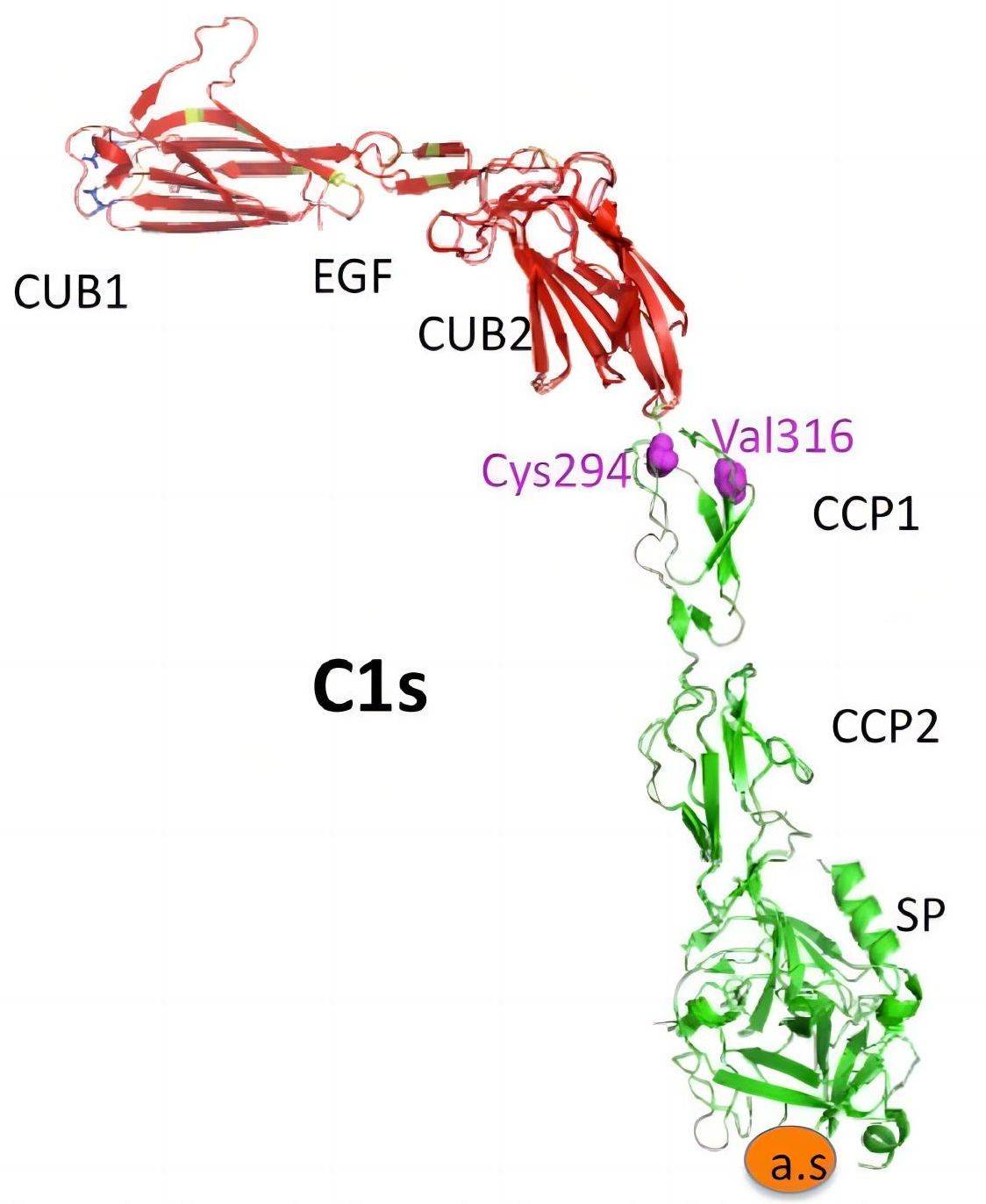
Fig. 1 The structure of C1s.1, 3
C1s Related Diseases
Dysregulation of C1s activity is implicated in a variety of pathological conditions. For instance, excessive C1s activity is a hallmark of Hereditary Angioedema (HAE), where a deficiency or dysfunction of C1 esterase inhibitor (C1-INH) leads to uncontrolled C1s activation and subsequent bradykinin generation, causing recurrent episodes of swelling. Beyond HAE, aberrant C1s activity contributes to the pathology of autoimmune diseases such as systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA), atypical Hemolytic Uremic Syndrome (aHUS), and conditions like ischemia-reperfusion injury and certain neurodegenerative disorders. Targeting C1s offers a highly specific therapeutic strategy to inhibit classical pathway activation at its earliest stage, thereby preventing downstream inflammatory and cytolytic effects while potentially preserving alternative and lectin pathway functions that are important for host defense.
Periodontal Ehlers-Danlos syndrome constitutes an autosomal dominant inherited condition marked by severe early-onset periodontitis combined with Ehlers-Danlos characteristics. It manifests joint hyperlaxity, dermal hyperelasticity, and tissue fragility. Mutations in collagen-associated genes—including the collagen-modifying enzymes lysyl hydroxylase and procollagen N-peptidase—underlie multiple EDS subtypes. Critically, C1R and C1S gene mutations cause pEDS. C1s is additionally associated with Hashimoto Disease, an autoimmune pathology targeting self-tissues and organs.
What We Can Offer?
Creative Biolabs is dedicated to providing a comprehensive range of products and services specifically designed to support your research and development efforts targeting Complement Therapeutic Target-C1s. Our offerings are crafted to accelerate your discovery process and ensure the highest quality outcomes.
-
Recombinant C1s Proteins: High-purity, active, and inactive forms of human and other species' C1s proteins for assay development, screening, and antibody generation.
-
Anti-C1s Antibodies: A diverse catalog of monoclonal and polyclonal antibodies targeting C1s, suitable for research, diagnostic, and potential therapeutic applications.
-
C1s Activity Assays: Robust and sensitive enzymatic and functional assays to measure C1s activity and screen for potential inhibitors.
-
C1s Inhibitor Screening Services: High-throughput screening platforms to identify novel small molecule or biologic inhibitors of C1s.
-
Custom Antibody Development: Bespoke antibody generation services, including hybridoma and phage display technologies, to develop highly specific antibodies against C1s.
-
Complement Pathway Profiling: Comprehensive analysis services to assess the impact of C1s modulation on overall complement pathway activation in various biological matrices.
-
Preclinical Efficacy Studies: In vitro and in vivo models to evaluate the therapeutic potential and safety of C1s-targeted agents.
Why Choose Us?
Choosing Creative Biolabs for your Complement Therapeutic Target-C1s projects provides you with unparalleled advantages rooted in our extensive experience and cutting-edge capabilities. Our dedication to scientific distinction and client achievement distinguishes us.
-
Deep Expertise: With over 20 years of specialized experience in complement biology and therapeutic development, our team possesses an in-depth understanding of C1s and its role in disease pathogenesis. This expertise translates into highly effective and targeted solutions for your unique project needs.
-
Advanced Platforms: We leverage state-of-the-art antibody discovery and engineering platforms, alongside high-throughput screening technologies, to identify and optimize C1s-specific therapeutic candidates with superior affinity and specificity.
-
Comprehensive Service Portfolio: From initial target validation and assay development to lead optimization and preclinical efficacy studies, we offer a seamless, integrated suite of services covering the entire therapeutic development pipeline for C1s inhibitors.
-
High-Quality Reagents: We provide meticulously characterized recombinant C1s proteins, highly specific anti-C1s antibodies, and validated functional assays, ensuring the reliability and reproducibility of your research.
-
Proven Track Record: Our success in developing precise complement therapeutics is supported by extensive internal data and Published Data, demonstrating our capability to deliver impactful results.
-
Customized Solutions: Recognizing that each project is unique, we offer highly flexible and customized service packages tailored to meet your specific research objectives and timelines.
Access the Creative Biolabs Edge - Obtain Your Quotation Now
Published Data
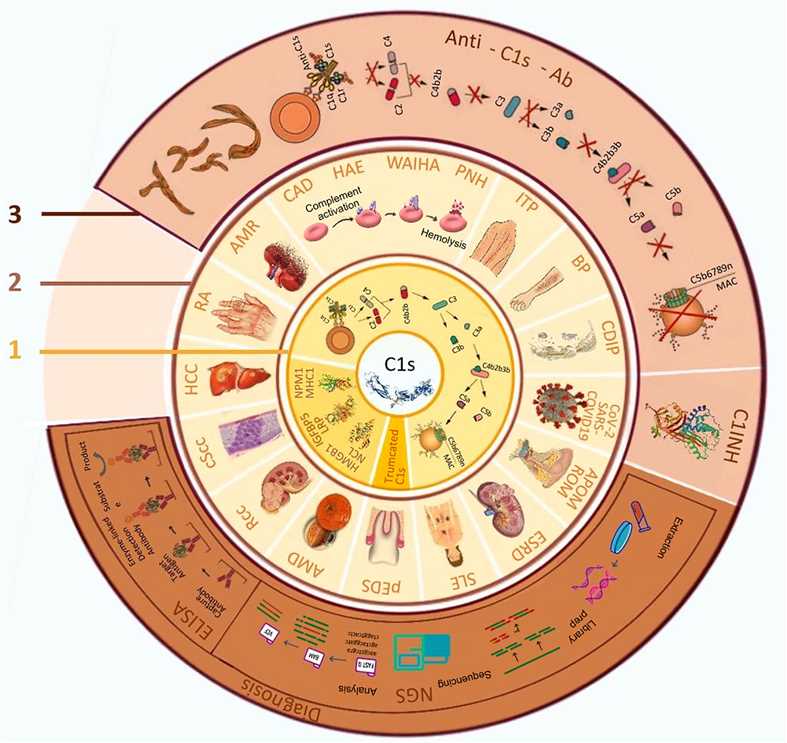 Fig.2 C1s: biological roles, associated disorders, and implications for diagnosis and therapy.2,3
Fig.2 C1s: biological roles, associated disorders, and implications for diagnosis and therapy.2,3
The complement system serves as a crucial link between innate and adaptive immune pathways, ensuring physiological equilibrium. Central to this system is the C1 complex, comprising C1q, C1r, and C1s, which activates the classical complement pathway. C1s deficiency is linked to early-onset systemic lupus erythematosus and an increased risk of bacterial infections. Conversely, gain-of-function mutations in C1r and C1s can cause periodontal Ehlers-Danlos syndrome. Given C1s’s role in inflammation, autoimmunity, and cancer, it’s a promising diagnostic and therapeutic biomarker. Therefore, advanced methods to measure C1s levels and activity are essential. Various small molecules, peptides, and monoclonal antibodies targeting C1s have been developed, including a US FDA-approved antibody for cold agglutinin disease, a type of autoimmune hemolytic anemia.
FAQs
Here are some common questions from researchers considering C1s as a therapeutic target:
Q: How can targeting C1s offer a more precise therapeutic approach compared to broader complement inhibitors?
A: Inhibiting C1s specifically blocks classical complement pathway initiation, preserving alternative and lectin pathways' functionality. This precision can reduce the risk of broad immunosuppression associated with more general complement inhibitors, potentially maintaining essential host defense mechanisms while mitigating pathological activation.
Q: What types of diseases are most amenable to C1s-targeted therapies?
A: Diseases driven primarily by classical complement pathway overactivation are excellent candidates. This includes conditions like Hereditary Angioedema, certain autoimmune diseases such as systemic lupus erythematosus and rheumatoid arthritis, atypical hemolytic uremic syndrome, and various inflammatory conditions where classical pathway activation contributes significantly to pathology.
Q: Are there specific considerations for developing C1s inhibitors that maintain broad complement function while preventing pathological activation?
A: Yes, a key consideration is achieving high specificity for C1s while avoiding off-target effects on other proteases or complement components. The goal is to selectively block the pathological activation of the classical pathway without compromising the immune system's ability to clear pathogens via other complement routes. This often involves careful design and rigorous in vitro and in vivo validation.
Q: How does the development process for C1s-specific antibodies differ from general antibody development?
A: While the foundational principles of antibody development remain similar, developing C1s-specific antibodies requires specialized expertise in complement biology. This includes using highly purified C1s antigens, employing functional assays to screen for inhibitory activity, and validating specificity within the complex environment of the complement cascade to ensure therapeutic efficacy and safety.
Q: What are the key advantages of focusing on C1s as a therapeutic target over other complement components?
A: Targeting C1s offers the advantage of inhibiting the classical pathway at its earliest stage, effectively preventing the entire downstream cascade of inflammation and tissue damage. This upstream intervention can be highly effective in diseases where classical pathway overactivation is the primary driver of pathology, potentially offering a more selective and potent therapeutic strategy.
Access the Creative Biolabs Edge – Request Your Quote Now
Related Hot Products
Featured Services
References
-
Kapferer-Seebacher, Ines, et al. "Periodontal Ehlers-Danlos syndrome is caused by mutations in C1R and C1S, which encode subcomponents C1r and C1s of complement." The American Journal of Human Genetics 99.5 (2016): 1005-1014. DOI:10.1016/j.ajhg.2016.08.019.
-
Ye, Jun, et al. "Complement C1s as a diagnostic marker and therapeutic target: Progress and propective." Frontiers in immunology 13 (2022): 1015128. DOI:10.3389/fimmu.2022.1015128.
-
Distributed under an Open Access license CC BY 4.0, without modification.
For Research Use Only.
Related Sections:


 Fig.2 C1s: biological roles, associated disorders, and implications for diagnosis and therapy.2,3
Fig.2 C1s: biological roles, associated disorders, and implications for diagnosis and therapy.2,3