Product List Background Clusterin-α Functional Service
Background
Clusterin, also known as CLU, is a multifunctional glycoprotein that aggregates blood cells in vitro and is found in nearly all body fluids and tissues, particularly in the central nervous system and endocrine tissues. Despite its established roles in cellular survival, pain, hunger, and cardiac protection, a comprehensive understanding of clusterin's functions still needs to be improved. Clusterin has potential as a prognostic biomarker and therapeutic target, covering biological synthesis and physiological roles. Canonical clusterin synthesis begins with mRNA transcription, producing a preprotein that undergoes glycosylation in the Golgi apparatus, ultimately forming a mature heterodimer consisting of α and β subunits linked by disulfide bonds.
Its Gene ID: 1191, UniProtKB ID: P10909, and OMIM ID: 185430.
Clusterin Secreted Signaling Pathway
Mature, secreted clusterin is produced from a 9-exon mRNA transcript, primarily translating from the ATG in exon 2. This translation makes a preproprotein targeted to the ER for subsequent post-translational modifications. The N-terminal ER-signal peptide is cleaved to form an immature proprotein, which is then phosphorylated and glycosylated in the ER and Golgi. Further cleavage in the Golgi generate α- and β-chains linked by disulfide bonds, resulting in a highly glycosylated heterodimer. Additionally, clusterin promotes apoptosis by interacting with the anti-apoptotic Bcl-xl protein on the mitochondrial membrane, affecting membrane permeability and releasing proapoptotic proteins.
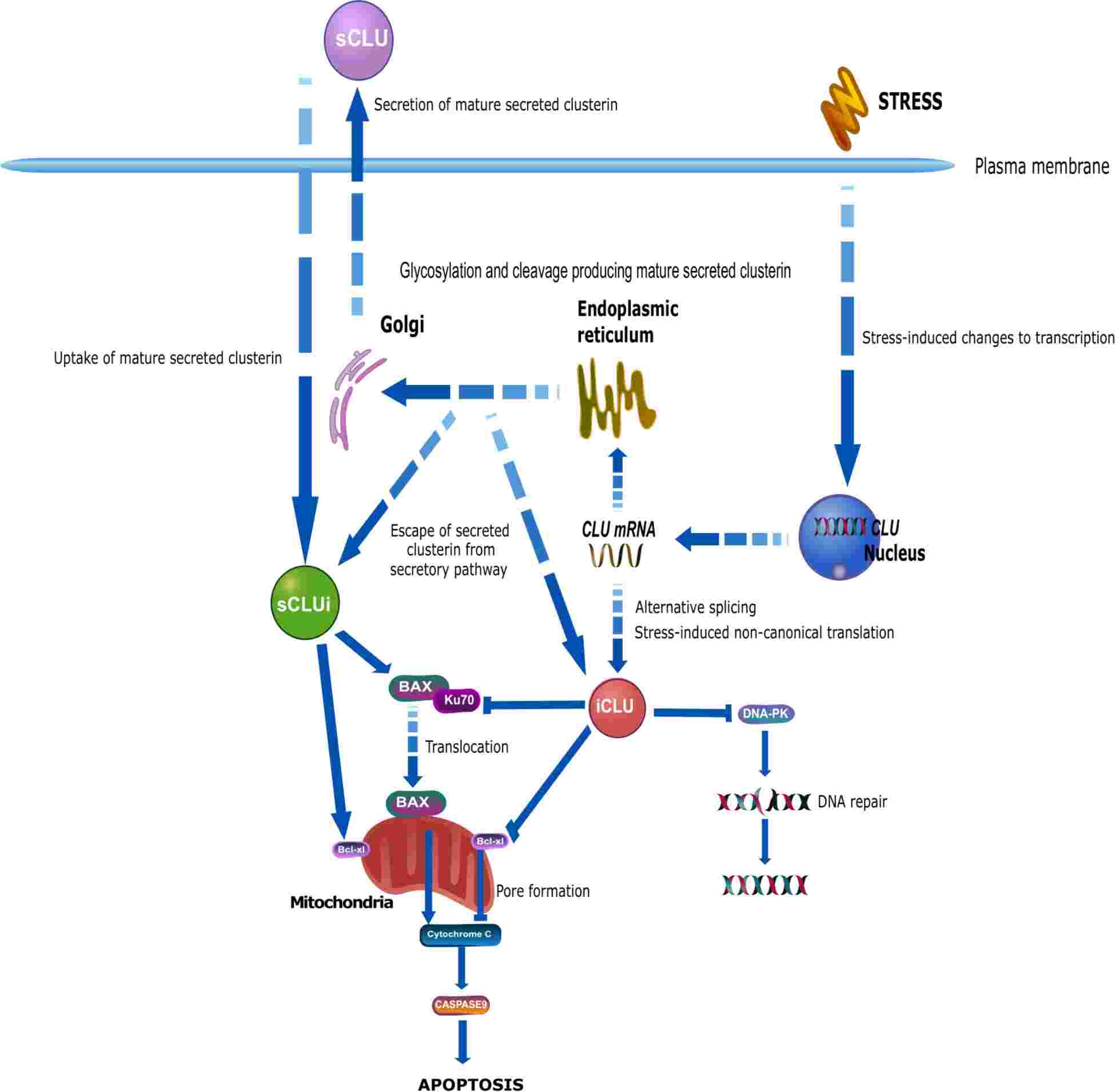 Fig.1 CD59 involved in complement pathways.1, 4
Fig.1 CD59 involved in complement pathways.1, 4
Functional Applications of Clusterin-α Products
Exploring Clusterin's Role in Overcoming Drug Resistance
The clinical efficacy of precision medicine is significantly hampered by de novo or acquired drug resistance, presenting a significant unmet clinical need. Tumor suppressor genes (TSGs) are crucial in tumorigenesis and influence therapeutic outcomes. Recent investigations employing in vitro cell lines, in vivo mouse models, and clinical data have elucidated the tumor-suppressive function of clusterin in lung cancer. Western blot and other assays using anti-clusterin antibodies delineate the signaling cascade triggered by clusterin loss of function in non-small cell lung cancer (NSCLC) cells. Mechanistically, clusterin inhibits TGFBR1, preventing the recruitment of the TRAF6/TAB2/TAK1 complex and subsequent activation of the TAK1-NF-κB signaling axis. Combination therapy with TAK1 and MEK1/2 inhibitors effectively reduces Kras mutation-positive and clusterin-deficient NSCLC tumors.
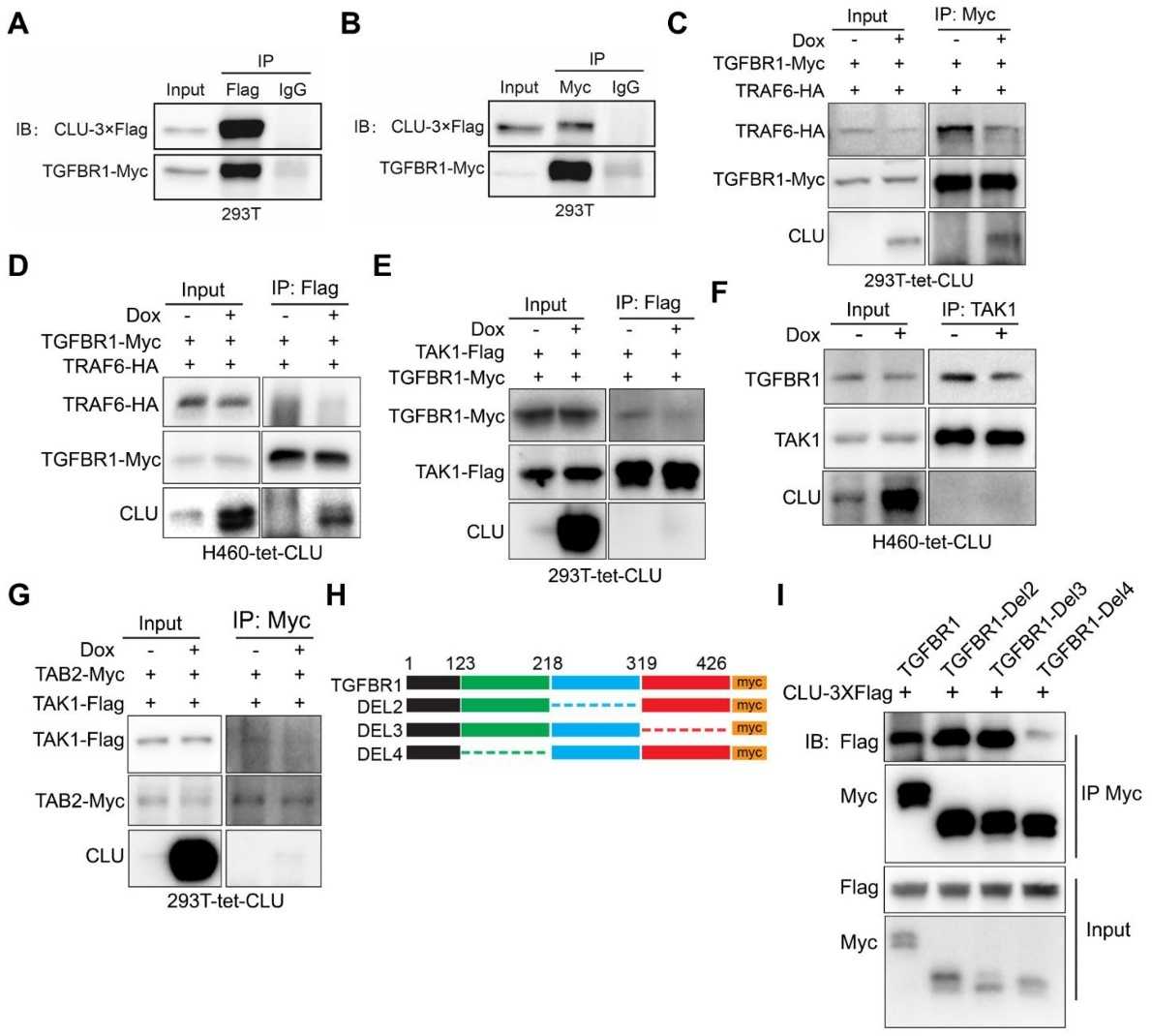 Fig.2 Interaction between TGFBR1 and clusterin demonstrated by reciprocal immunoprecipitation.2, 4
Fig.2 Interaction between TGFBR1 and clusterin demonstrated by reciprocal immunoprecipitation.2, 4
Mitochondrial Dynamics Modulation by Clusterin in Diabetes-Induced Endothelial Dysfunction
Clusterin, known for its stress-response functions, is pivotal in mitigating diabetes-induced endothelial dysfunction. Utilizing diabetes models, researchers have investigated clusterin's impact and underlying mechanisms in this context. Immunohistochemical analysis using antibodies against clusterin-α has demonstrated that its overexpression reduces ICAM-1/VCAM-1 expression in aortic tissues and enhances endothelium-dependent vasodilation in diabetic mice. In vitro, studies have revealed that adenoviral-mediated clusterin overexpression attenuates pro-inflammatory cytokine expression and inhibits monocyte adhesion to endothelial cells exposed to high glucose and palmitate. Mechanistically, clusterin overexpression suppresses mitochondrial excessive fission and reduces mitochondrial ROS production. Conversely, clusterin silencing exacerbates mitochondrial fission and endothelial inflammatory responses under high glucose conditions. These findings highlight clusterin's potential as a therapeutic target to mitigate vascular complications of diabetes by modulating mitochondrial dynamics through AMP-activated protein kinase (AMPK) activation.
Creative Biolabs offers a comprehensive range of clusterin-α-targeted products, including assay kits, recombinant proteins, and anti-clusterin-α antibodies. We also provide custom services to develop clusterin-α-specific products, such as bespoke clusterin-α-based bispecific antibody solutions tailored to meet individual needs.
Clusterin-α Functional Service
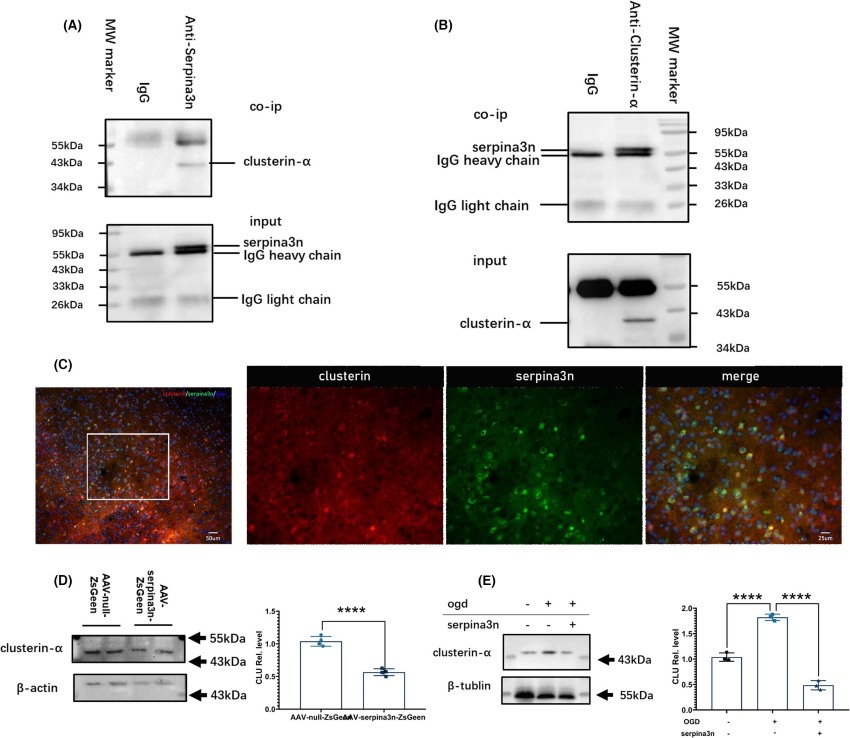 Fig.3 Clusterin-α as a protein interacting partner of SerpinA3N.3, 4
Fig.3 Clusterin-α as a protein interacting partner of SerpinA3N.3, 4
To investigate the role of serine protein inhibitor A3N (serpinA3N) in the context of ischemic stroke, researchers explored its interaction with clusterin-α. Through co-immunoprecipitation (Co-IP) assays, the binding of serpinA3N to clusterin-α was confirmed. Immunofluorescence double-staining revealed colocalization of serpinA3N and clusterin-α in cortical neurons following stroke. Subsequent Western blot analysis indicated that serpinA3N overexpression decreased clusterin-α levels both post-middle cerebral artery occlusion and in neurons after oxygen-glucose deprivation/reoxygenation. Additionally, serpinA3N was found to upregulate Akt-mTOR pathway members, reducing neuronal apoptosis and neuroinflammation. Consequently, serpinA3N may mitigate brain injury post-stroke through its interaction with clusterin-α.
Creative Biolabs provides specialized services focused on clusterin-α functionality, encompassing the examination of clusterin-α interaction dynamics and additional functional assessments. These services are precisely tailored to meet the specific requirements of esteemed clients engaged in clinical and scientific research domains.
References
-
Foster, Evangeline M., et al. "Clusterin in Alzheimer’s disease: mechanisms, genetics, and lessons from other pathologies." Frontiers in neuroscience 13 (2019): 164.
-
Chen, Zhipeng, et al. "Inactivation of tumor suppressor gene Clusterin leads to hyperactivation of TAK1-NF-κB signaling axis in lung cancer cells and denotes a therapeutic opportunity." Theranostics 10.25 (2020): 11520.
-
Zhang, Yu, et al. "SerpinA3N attenuates ischemic stroke injury by reducing apoptosis and neuroinflammation." CNS Neuroscience & Therapeutics 28.4 (2022): 566-579.
-
Distributed under Open Access license CC BY 4.0, without modification.


 Datasheet
Datasheet Fig.1 CD59 involved in complement pathways.1, 4
Fig.1 CD59 involved in complement pathways.1, 4
 Fig.2 Interaction between TGFBR1 and clusterin demonstrated by reciprocal immunoprecipitation.2, 4
Fig.2 Interaction between TGFBR1 and clusterin demonstrated by reciprocal immunoprecipitation.2, 4
 Fig.3 Clusterin-α as a protein interacting partner of SerpinA3N.3, 4
Fig.3 Clusterin-α as a protein interacting partner of SerpinA3N.3, 4
